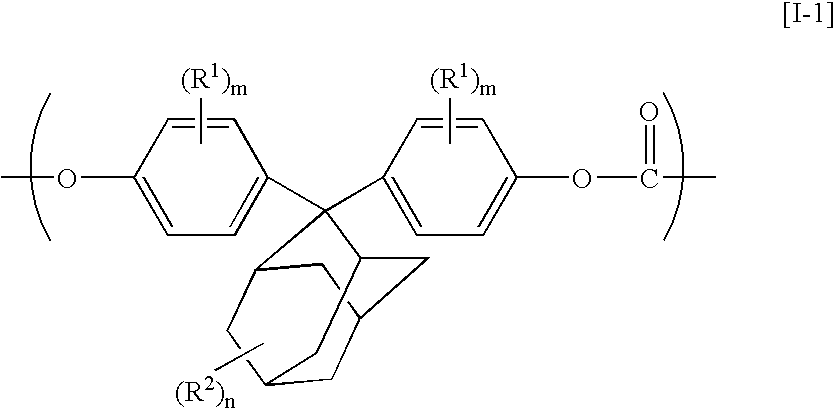
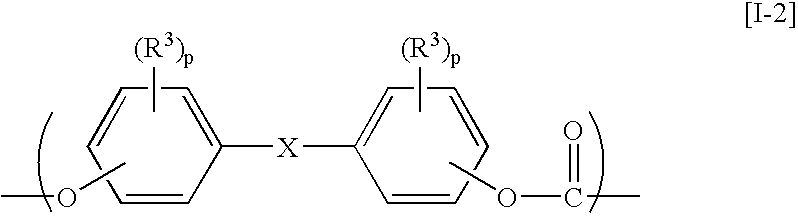
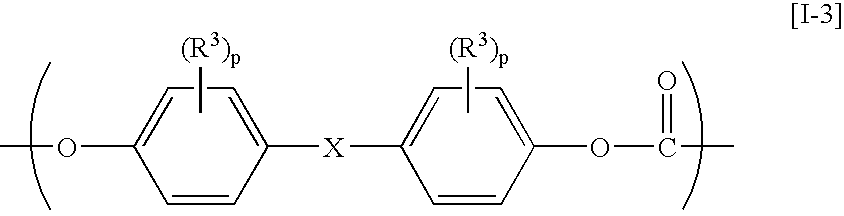
Aromatic polycarbonate resin, process for producing the same, optical-part molding material, and optical part
a technology of aromatic polycarbonate resin and production process, applied in the direction of manufacturing tools, instruments, lenses, etc., can solve the problems of poor solvent solubility, inferior moldability, and reduced transparency of molded articles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i-1
[0088]Methylene chloride 700 ml which was a solvent was added to a solution prepared by dissolving 45 g of 2,2-bis(4-hydroxyphenyl)adamantane and 25 g of 1,1-bis(4-hydroxyphenyl)cyclohexane in 1,360 ml of a potassium hydroxide aqueous solution having a concentration of 2 normal, and phosgene gas was blown into the above solution for 30 minutes in a proportion of 950 ml / minute under cooling while stirring. Then, this reaction liquid was left standing still and separating, and a methylene chloride solution of an oligomer having a polymerization degree of 2 to 5 and having a chloroformate group at a molecular end was obtained in the organic layer.
[0089]Methylene chloride was added to 110 ml of the methylene chloride solution thus obtained to control the whole amount to 150 ml, and then a solution prepared by dissolving 5 g of 1,1-bis(4-hydroxyphenyl)cyclohexane in 50 ml of a potassium hydroxide aqueous solution having a concentration of 2 normal was added thereto. Further, 0.2 g of p-t...
example i-2
[0094]Methylene chloride 700 ml which was a solvent was added to a solution prepared by dissolving 75 g of 2,2-bis(4-hydroxyphenyl)adamantane in 1,360 ml of a potassium hydroxide aqueous solution having a concentration of 2 normal, and phosgene gas was blown into the above solution for 30 minutes in a proportion of 950 ml / minute under cooling while stirring. Then, this reaction liquid was left standing still and separating, and a methylene chloride solution of an oligomer having a polymerization degree of 2 to 5 and having a chloroformate group at a molecular end was obtained in the organic layer.
[0095]Methylene chloride was added to 110 ml of the methylene chloride solution thus obtained to control the whole amount to 150 ml, and then a solution prepared by dissolving 6 g of 9,9-bis(3-methyl-4-hydroxyphenyl)fluorene in 50 ml of a potassium hydroxide aqueous solution having a concentration of 2 normal was added thereto. Further, 0.2 g of p-tert-butylphenol was added thereto as a mol...
example i-3
[0100]Methylene chloride 900 ml which was a solvent was added to a solution prepared by dissolving 170 g of 1,1-bis(4-hydroxyphenyl)cyclohexane in 1,530 ml of a potassium hydroxide aqueous solution having a concentration of 2 normal, and phosgene gas was blown into the above solution for 30 minutes in a proportion of 950 ml / minute under cooling while stirring. Then, this reaction liquid was left standing still and separating, and a methylene chloride solution of an oligomer having a polymerization degree of 2 to 5 and having a chloroformate group at a molecular end was obtained in the organic layer.
[0101]Methylene chloride was added to 110 ml of the methylene chloride solution thus obtained to control the whole amount to 150 ml, and then a solution prepared by dissolving 6 g of 2,2-bis(3,5-dimethyl-4-hydroxyphenyl)adamantane in 50 ml of a potassium hydroxide aqueous solution having a concentration of 2 normal was added thereto. Further, 0.2 g of p-tert-butylphenol was added thereto ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| mole ratio | aaaaa | aaaaa |
| mole ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com