Hyaluronic acid- cyclodextrin-adamantane polyethylene glycol carrier as well as preparation method and application thereof
A technology of hyaluronic acid and polyethylene glycol, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of normal cell damage and limit the clinical application of chemotherapy drugs, etc. Achieve the effect of increasing long cycle time, easy operation and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Synthesis of hyaluronic acid-hydroxypropyl-β-cyclodextrin (HA-HP-β-CD) (cyclodextrin substitution degree is 20%)
[0036] Hyaluronic acid (0.2g, -COOH, 0.62mM, M=7000) was dissolved in 50mL of anhydrous formamide, heated in a 40°C water bath with magnetic stirring to dissolve, and cooled to room temperature. EDC (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride) (0.2 g) was added and magnetically stirred for 3 h. Then HP-β-CD (0.22g, -OH, 1.8mM) was dissolved in 5mL of anhydrous formamide, and slowly added dropwise to the HA mixture. The addition was completed within 10 minutes, and stirring was continued at room temperature for 24h. The reaction mixture was dialyzed in distilled water for 2 days, filtered, and freeze-dried to obtain a white powder.
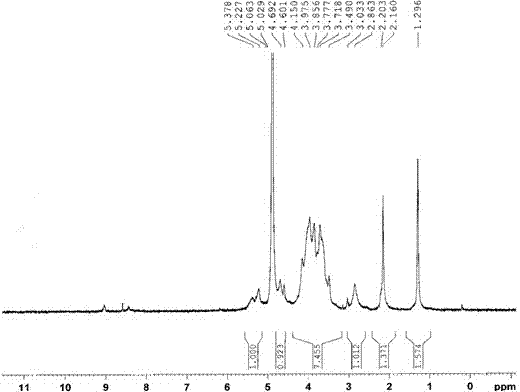
[0037] Determination by NMR 1 HNMR hydrogen spectrum is determined the structure of embodiment 1 compound, and the solvent of selection is D 2 O, the result is as figure 1 . 5.3-5.2ppm is H in hyd...
Embodiment 2
[0038] Example 2 Synthesis of hyaluronic acid-hydroxypropyl-β-cyclodextrin (HA-HP-β-CD) (the degree of substitution of cyclodextrin is 40%)
[0039] Hyaluronic acid (0.2g, -COOH, 0.62mM, M=7000) was dissolved in 50mL of anhydrous formamide, heated in a 40°C water bath with magnetic stirring to dissolve, and cooled to room temperature. EDC (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride) (0.2 g) was added and magnetically stirred for 3 h. Then HP-β-CD (0.4g, -OH, 3.5mM) was dissolved in 5mL of anhydrous formamide, and slowly added dropwise to the HA mixture. The addition was completed within 10 minutes, and stirring was continued at room temperature for 24h. The reaction mixture was dialyzed in distilled water for 2 days, filtered, and freeze-dried to obtain a white powder.
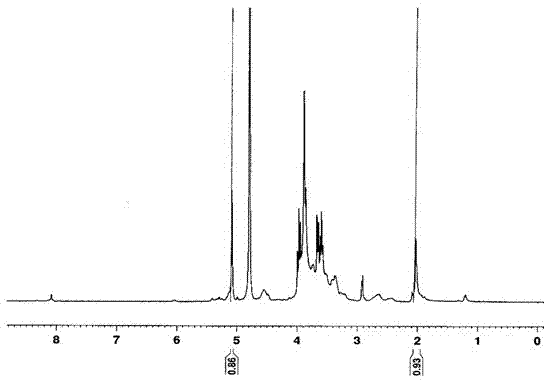
[0040] Determination by NMR 1 HNMR hydrogen spectrum is determined the structure of embodiment 1 compound, and the solvent of selection is D 2 O, 5.3-5.2ppm is H in hydroxypropyl cyclodextr...
Embodiment 3
[0041] Example 3 Synthesis of hyaluronic acid-hydroxypropyl-β-cyclodextrin (HA-HP-β-CD) (the degree of substitution of cyclodextrin is 50%)
[0042] Hyaluronic acid (0.2g, -COOH, 0.62mM, M=7000) was dissolved in 50mL of anhydrous formamide, heated in a 40°C water bath with magnetic stirring to dissolve, and cooled to room temperature. EDC (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride) (0.2 g) was added and magnetically stirred for 3 h. Then HP-β-CD (1.5g, -OH, 6.6mM) was dissolved in 5mL of anhydrous formamide, and slowly added dropwise to the HA mixture. The addition was completed within 10 minutes, and stirring was continued at room temperature for 24h. The reaction mixture was dialyzed in distilled water for 2 days, filtered, and freeze-dried to obtain a white powder.
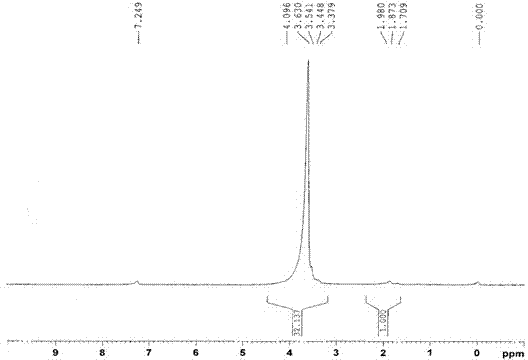
[0043] Determination by NMR 1 HNMR hydrogen spectrum is determined the structure of embodiment 1 compound, and the solvent of selection is D 2 O. 5.2-5.1ppm is H in hydroxypropyl cyclodext...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com