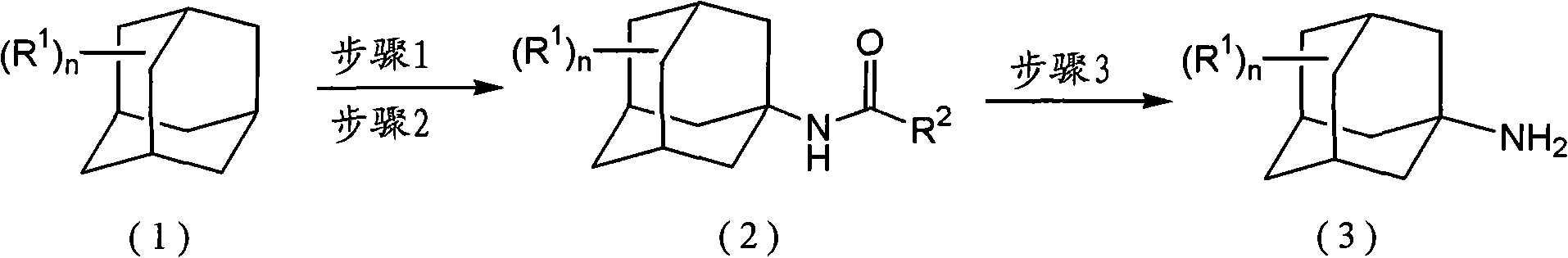
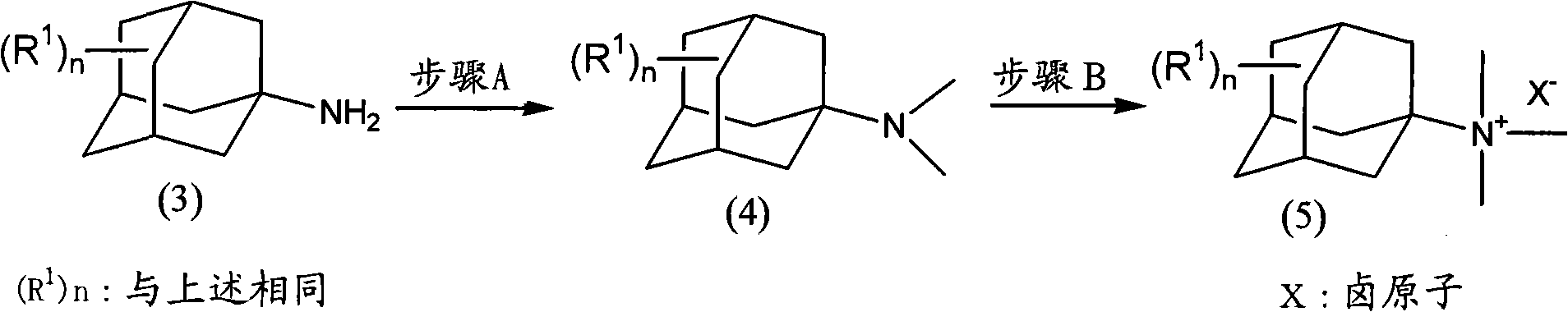
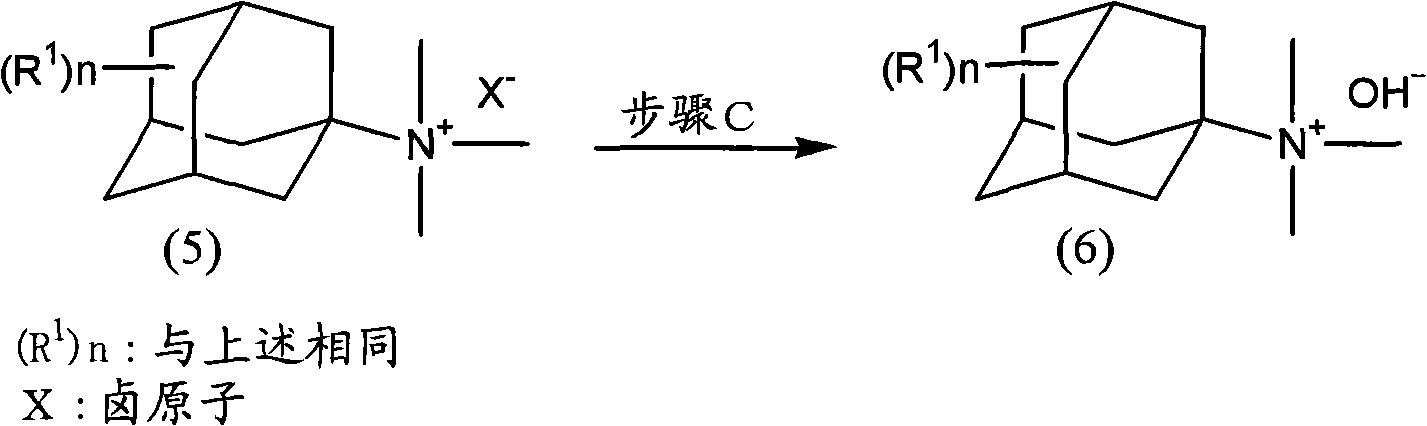
Method for producing amine series and quaternary ammonium salts with adamantane framework
A manufacturing method, adamantane technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino compounds from amines, etc., can solve the problem of slow reaction speed, difficult to purify, unable to obtain the target product with high purity and high yield, etc. problem to achieve high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0141] [Manufacturing Example 1]: Steps 1 and 2
[0142] 100 mL (1937 mmol) of 25 mass % oleum was placed in a 300 mL four-necked flask, 8 g (195 mmol) of acetonitrile and 20 g (147 mmol) of adamantane were added, and the mixture was reacted at a reaction temperature of 25° C. for 3 hours. In addition, the molar ratio of oleum to the raw material adamantane was 13.2 (=1937 / 147).
[0143] Next, the reaction liquid was added dropwise to 300 g of water at 5° C. placed in a 1 L four-necked flask. At the end of the dropwise addition, the temperature of the liquid mixture of water and the reaction liquid was 10°C. After completion of the dropwise addition, stirring was carried out at 10° C. for 30 minutes to complete the hydrolysis, and the precipitated crystals were filtered, washed with water, and dried to obtain 27.5 g of crystals.
[0144] According to gas chromatography analysis, the crystals were N-(1-adamantyl)acetamide with a GC purity of 98%, and the yield was 97 mol%.
manufacture example 2
[0145] [Manufacturing Example 2]: Steps 1 and 2
[0146] Except having changed the amount of acetonitrile to 12 g, the same operation as in Production Example 1 was performed. As a result, 27.8 g of N-(1-adamantyl)acetamide was obtained (98 mol% yield, 98% GC purity).
manufacture example 3
[0147] [Manufacturing Example 3]: Steps 1 and 2
[0148] Except having changed the reaction temperature from 25 degreeC to 40 degreeC, the same operation as manufacture example 1 was performed. As a result, 25.5 g of N-(1-adamantyl)acetamide was obtained (yield 90 mol%, GC purity 95%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| relative permittivity | aaaaa | aaaaa |
| relative permittivity | aaaaa | aaaaa |
| relative permittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com