Crf2 ligands in combination therapy
A technology of CRF2 and CRF1, which is applied in the field of CRF2 ligands for combination therapy, can solve problems such as confusion, decline, oligonucleotide toxicity and side effects, and body weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
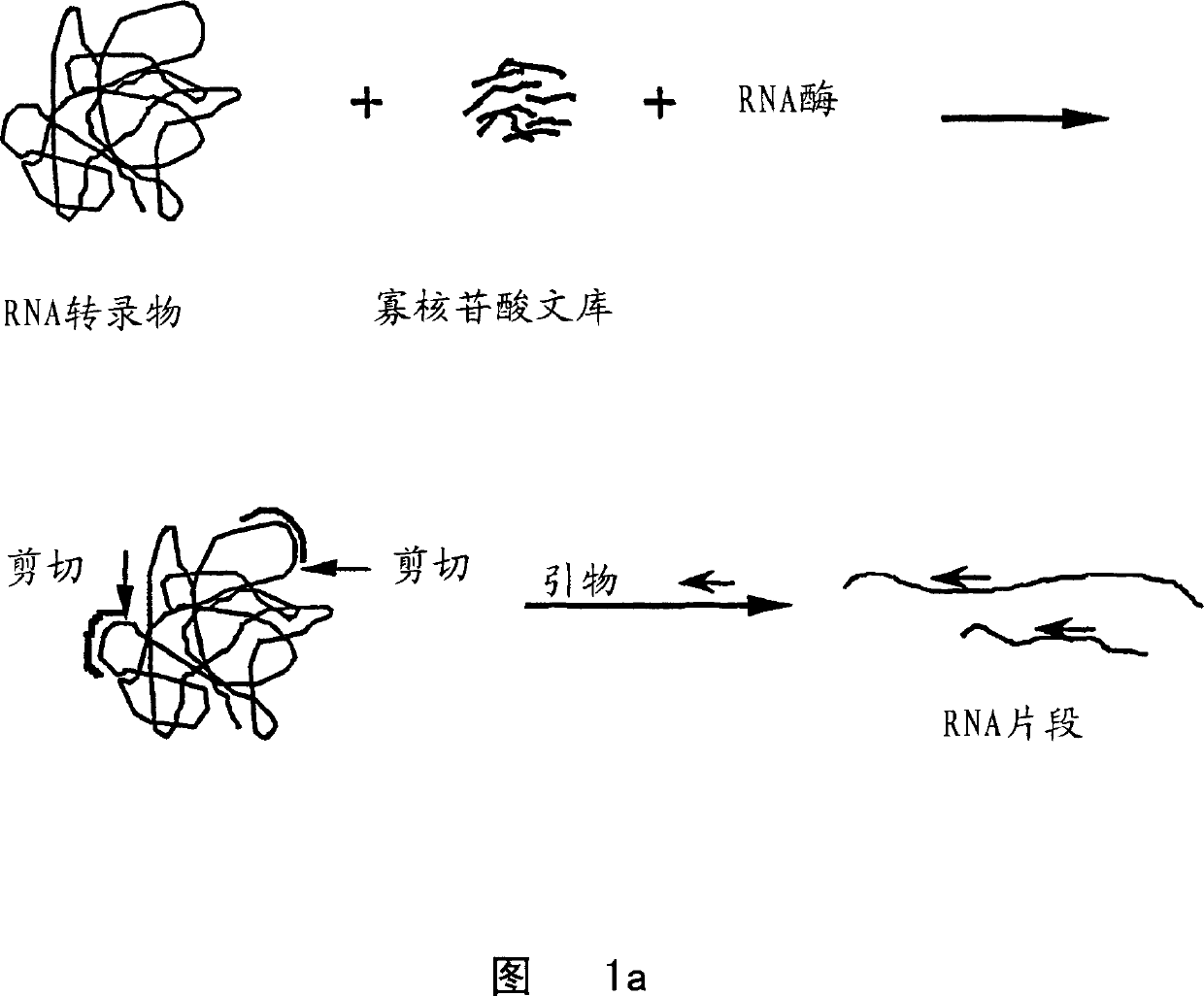
[0129] Synthesis and purification of oligonucleotides for in vivo experiments
[0130] Oligonucleotides were synthesized with an automated ABI 394 RNA / DNA synthesizer using standard synthesis protocols. The antisense and mismatch oligonucleotides used in the experiments shown in Figures 3 and 4 consisted of the following sequences:
[0131] Antisense: TGA CGC agc ggc acC AGA CC
[0132] Mismatches: TGA GGC acc gga acC ACA CC
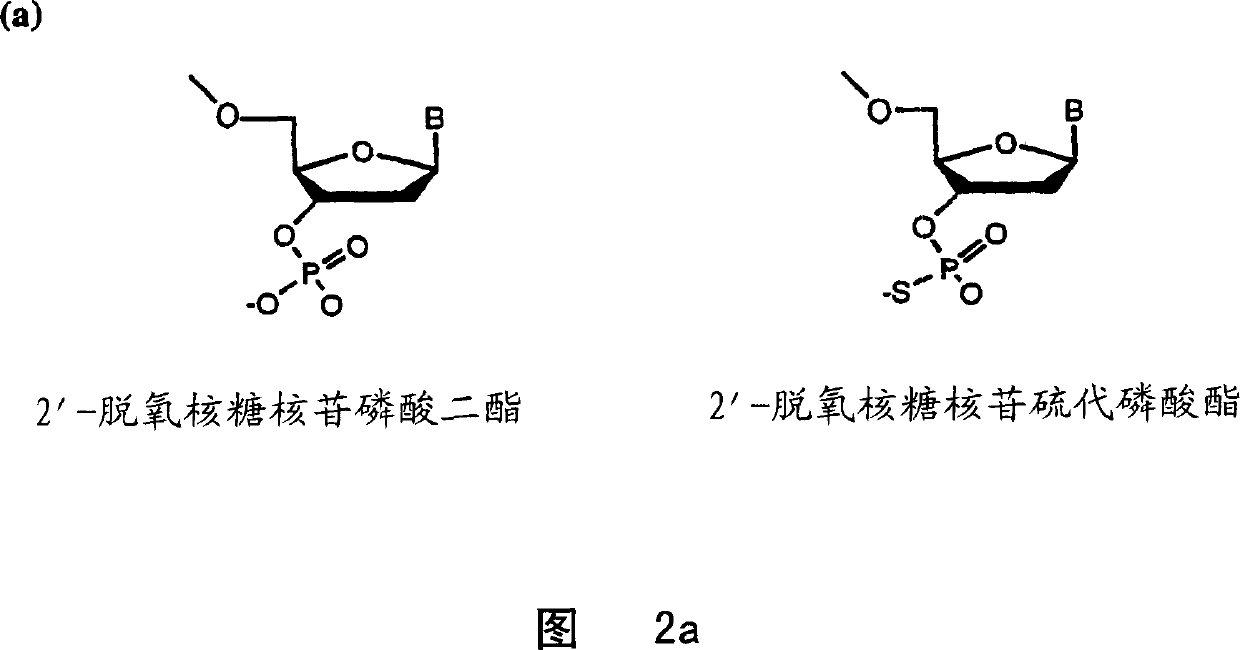
[0133] Wherein, uppercase letters indicate 2'-methoxyribonucleoside phosphodiester residues, while lowercase letters indicate 2'-deoxyribonucleoside phosphorothioate residues. 2'-Methoxyribonucleoside phosphoimidate was purchased from Chem Genes, propynyl and 5-methylcytidine phosphoimidate were obtained from Glen Research, and 2'-fluoro phosphoimido Esters were obtained from NeXstar. Beaucage reagent for synthesis of phosphorothioate linkages and fluorescein phosphoramidite for 5' labeling of oligonucleotides were purchased from Glen Research. Th...
Embodiment 2
[0136] Animals and Surgery
[0137] Male Sprague Dawley rats (Charles River) weighing 320-360 grams at the time of surgery were housed individually in stainless steel cages and provided free access to food and water. After a 4-day acclimatization period, under anesthesia with Rompun (100 mg / kg) and ketamine (9 mg / kg), long-term 26-gauge catheters were implanted bilaterally in the rats, with the catheters positioned in the lateral ventricle . Orientation coordinates were: incision line 3.3 mm below the interauricular line; 0.2 mm posterior to the brine; ±2.7 mm lateral to the midline; 3.8 mm ventral to the skull surface, and an angle of 24°. A syringe (33 gauge) protruded 0.5 mm beyond the catheter tip. The animals were subjected to daily acclimatization controls beginning 2 days after surgery.
[0138] All animal care used published methods approved by the Institutional Animal Care and Use Committee (IACUC). DuPont Pharmaceutical Research Laboratories is accredited by th...
Embodiment 3
[0140] oligonucleotide administration
[0141] Oligonucleotide infusions were started on day 8 after surgery when the rats weighed approximately 20 grams higher than the weight at surgery. Fresh oligonucleotide solutions were prepared daily by dissolving lyophilized oligonucleotide particles in sterile saline. Rats were weighed daily at 9 am prior to infusion with oligonucleotides. 1 microliter of the solution was injected into each ventricle over 2 minutes using a microprocessor controlled syringe pump (Stoelting). The syringes for each rat were rinsed with ethanol and sterile water and dried between daily injections.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com