Transluminal drug delivery methods and devices
a technology of transluminal drug delivery and delivery method, which is applied in the direction of prosthesis, biocide, heterocyclic compound active ingredients, etc., can solve the problems of inability to use oral anesthetic agents to inhibit urinary tract sensory nerve activation, limited treatment options for ic, and growing healthcare problems in the world
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
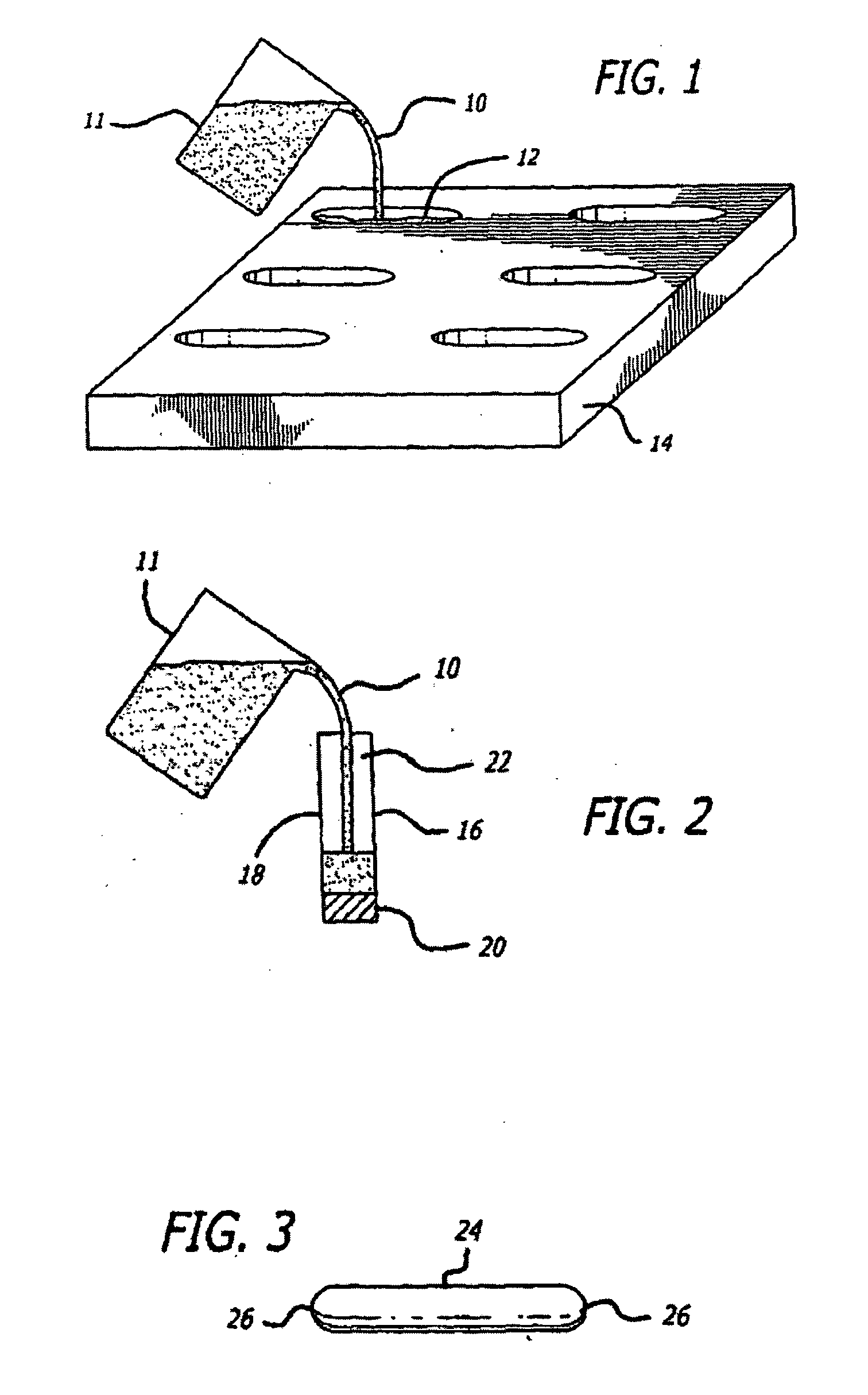
[0134]Suppository Fabrication
[0135]Animal tests, using a New Zealand White rabbit model, were conducted to determine the safety profile of buffered lidocaine absorption by measuring plasma levels of lidocaine in the systemic circulation using different carrier bases. Levels of lidocaine that would be toxic if injected intravenously were fabricated in a suppository carrier base. Different carrier bases were warmed in a 40° C. base and lidocaine was added in increments of 10% of the final weight of the mixture until it reached a ratio of 3:7 lidocaine to carrier base. The pH of the mixture was measured after all lidocaine had dissolved using a temperature compensating pH meter. A 30% lidocaine mixture was found to be the highest concentration lidocaine mixture achievable with the carrier base materials that were tried. The mixture was raised to 70° C. in a water bath and titrated with sodium bicarbonate to a pH of 8.5 while the mixture was gently stirred. Heating and stirring were sto...
example 2
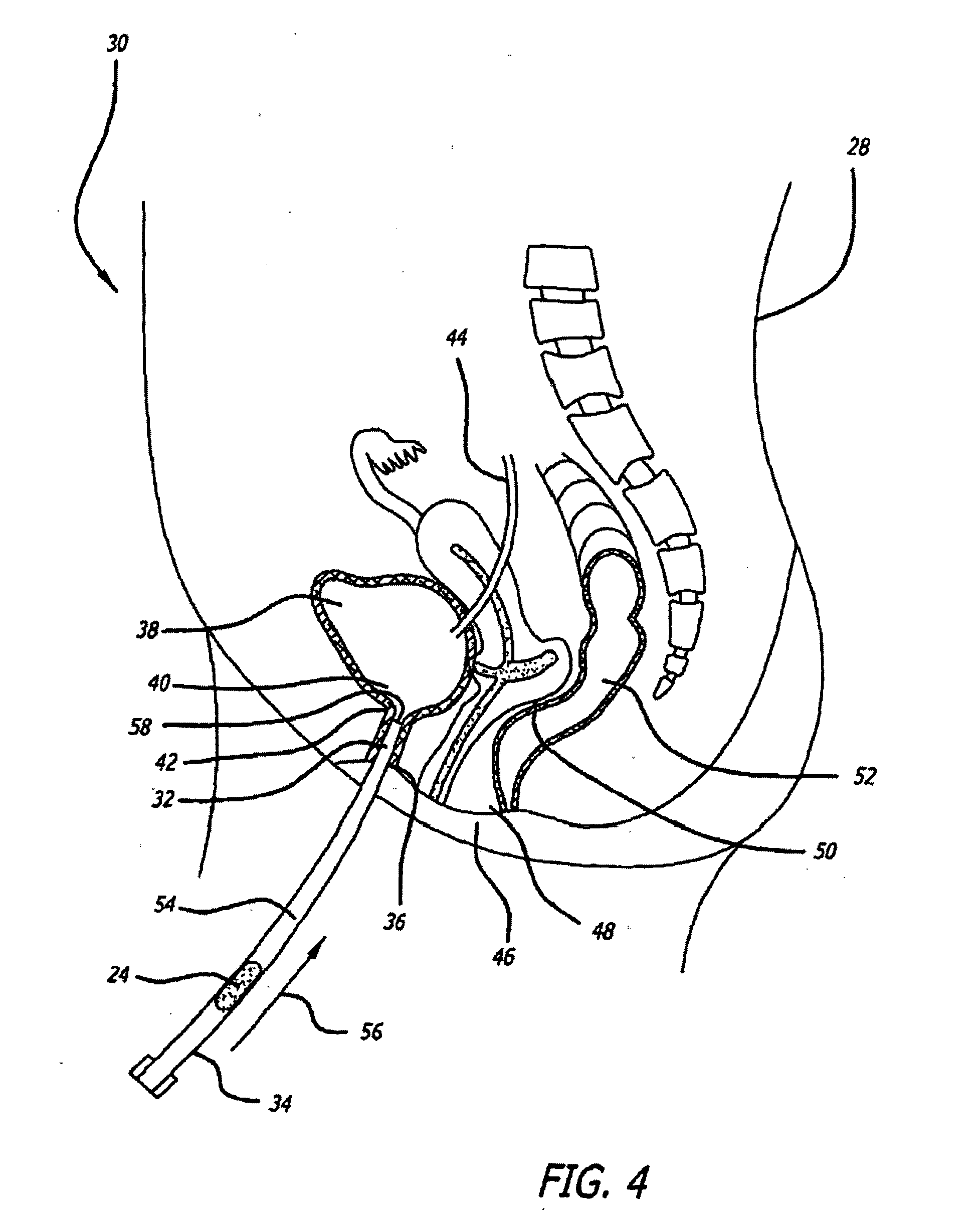
[0141]Human clinical studies were conducted to determine the efficacy of a buffered lidocaine suppository having a mucopolysaccharide component to repair any defect or injury to a luminal surface of a patient's urethra. A conical suppository with a weight of approximately 500 mg was used in the study. Suppositories were fabricated to contain approximately 10% lidocaine buffered to a pH of about 7.8. Methyl butyl ketone (MBK) was chosen as the suppository carrier base material, because the melting time of the base material could be adjusted by addition of paraffin to a melting time within a range of 5-15 minutes. Suppositories were fabricated with PEG but without any active ingredient to determine if the inflammatory response observed in the animals resulted in any adverse effects in humans. Suppositories containing PEG carrier base materials were placed in two human subjects with both subjects complaining of urethral burning and urinary frequency. As a result, PEG was not used clini...
example 3
[0144]The study of Example 3 investigates the amount of sodium bicarbonate necessary to buffer a lidocaine solution to a pH of 7.6-7.8. Sodium bicarbonate in 3 mg increments was added to three concentrations of lidocaine in two volumes of water. The three concentrations of lidocaine were 30, 45, and 60 mg. The rationale for this choice of lidocaine concentrations was they represent a 5-12% range of lidocaine concentrations, by weight, in the 500 mg suppository expected to be used in future clinical studies. This range of lidocaine concentrations in the suppository covers what will most likely be the lidocaine concentration used in the final clinical version of a buffered lidocaine suppository.
[0145]To measure pH, the lidocaine and sodium bicarbonate must be dissolved in solution. However, lidocaine and sodium bicarbonate are not dissolved in the suppository. Rather, they are in suspension in the carrier base and dissolve only after they are released from the carrier base and exposed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com