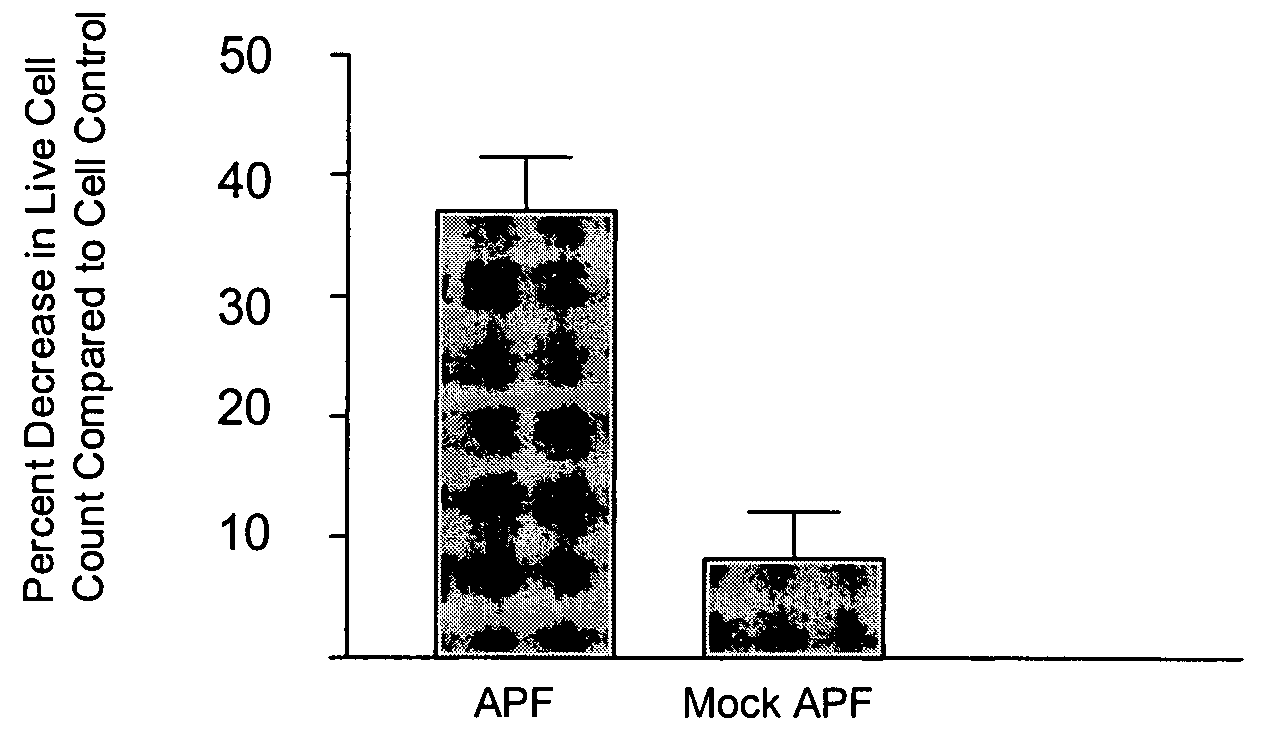
Novel antiproliferative factor and methods of use
a technology of antiproliferative factor and anti-proliferative agent, which is applied in the field of biochemistry, cell biology, chemistry, can solve the problems of loss of epithelial barrier integrity, unfavorable conventional methods of structural analysis, such as nmr spectroscopy, and achieve the effect of improving sensitivity to a cancer therapy, facilitating or facilitating the overcoming of resistan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Examplary Methods and Reagents
Cell Cultures
[0199] Cystoscopy was performed under general anesthesia, and 4 mm2 pieces of transitional epithelium with submucosa bladder tissue were obtained using rigid cold cup biopsy forceps from six patients who had previously undergone cystoscopy and fulfilled the NIDDK / NIH diagnostic criteria for interstitial cystitis, and six age- race- and gender-matched controls who were asymptomatic for urinary tract disease, as previously described (Keay et al., 2001; Keay et al., 2000; Keay et al., 2003a; Keay et al., 2003b; Keay et al., 1996). All patients were at least 18 years old and enrolled in accordance with guidelines of the Institutional Review Board of the University of Maryland School of Medicine.
[0200] Explanted epithelial cells were propagated from these biopsy specimens in DMEM-F12 (Media Tech, Herndon, Va.) with 10% heat inactivated fetal bovine serum (FBS), 1% antibiotic / antimycotic solution, 1% L-glutamine, 1.0 U / ml insulin (all from Si...
example 2
Identification of Amino Acids by Mass Spectometric Analysis
[0221] In specific aspects of the invention, an APF molecule is identified and / or characterized by any suitable means in the art, such as through the characterization of the peptide, sugar, or glycopeptide moiety of APF. In one specific embodiment, mass spectrometry is utilized. In other embodiments, techniques such as nuclear magnetic resonance or proteomic techniques (including isotope-coded affinity assays), and sensitive chromatographic methods may be utilized, for example.
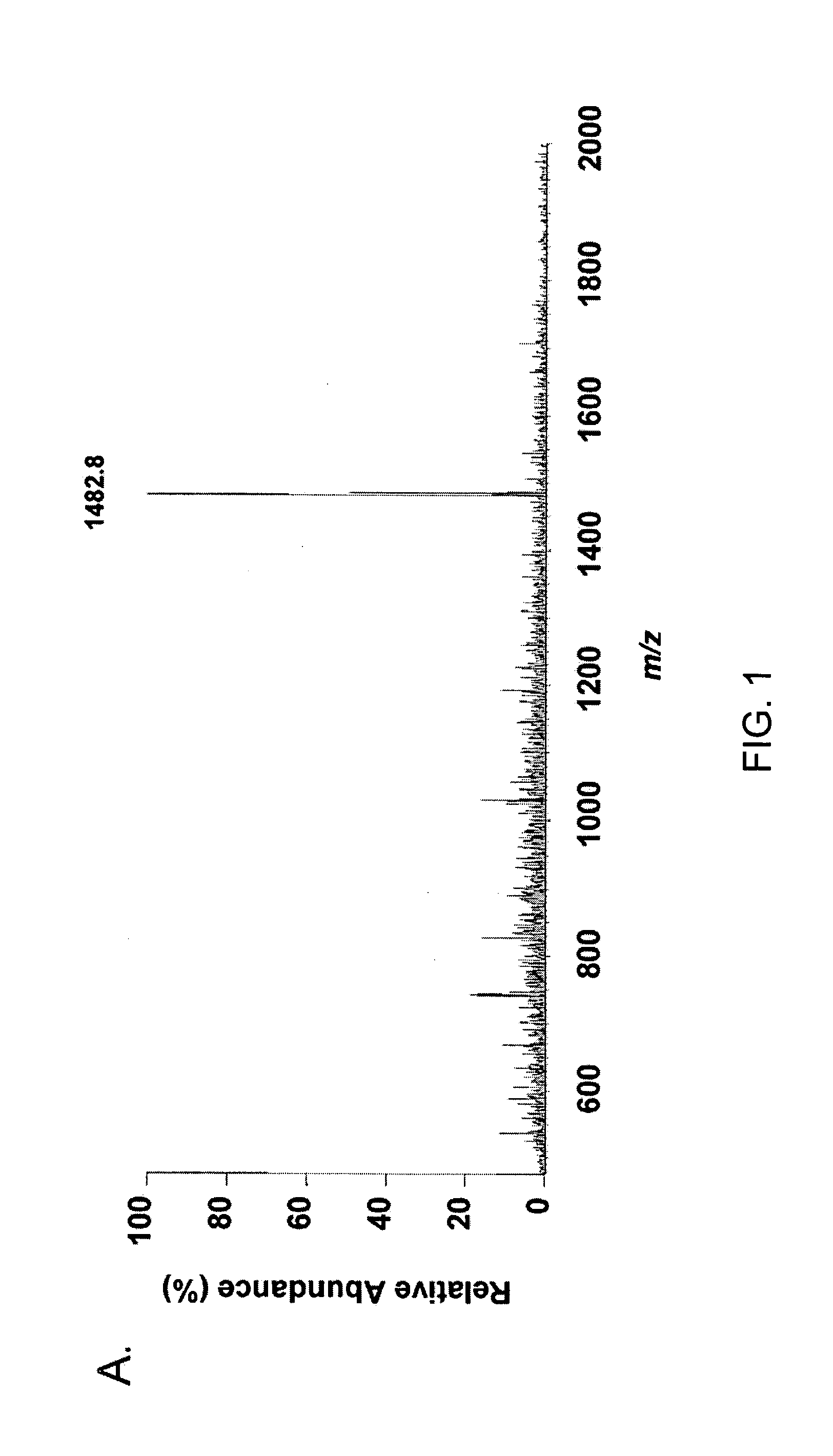
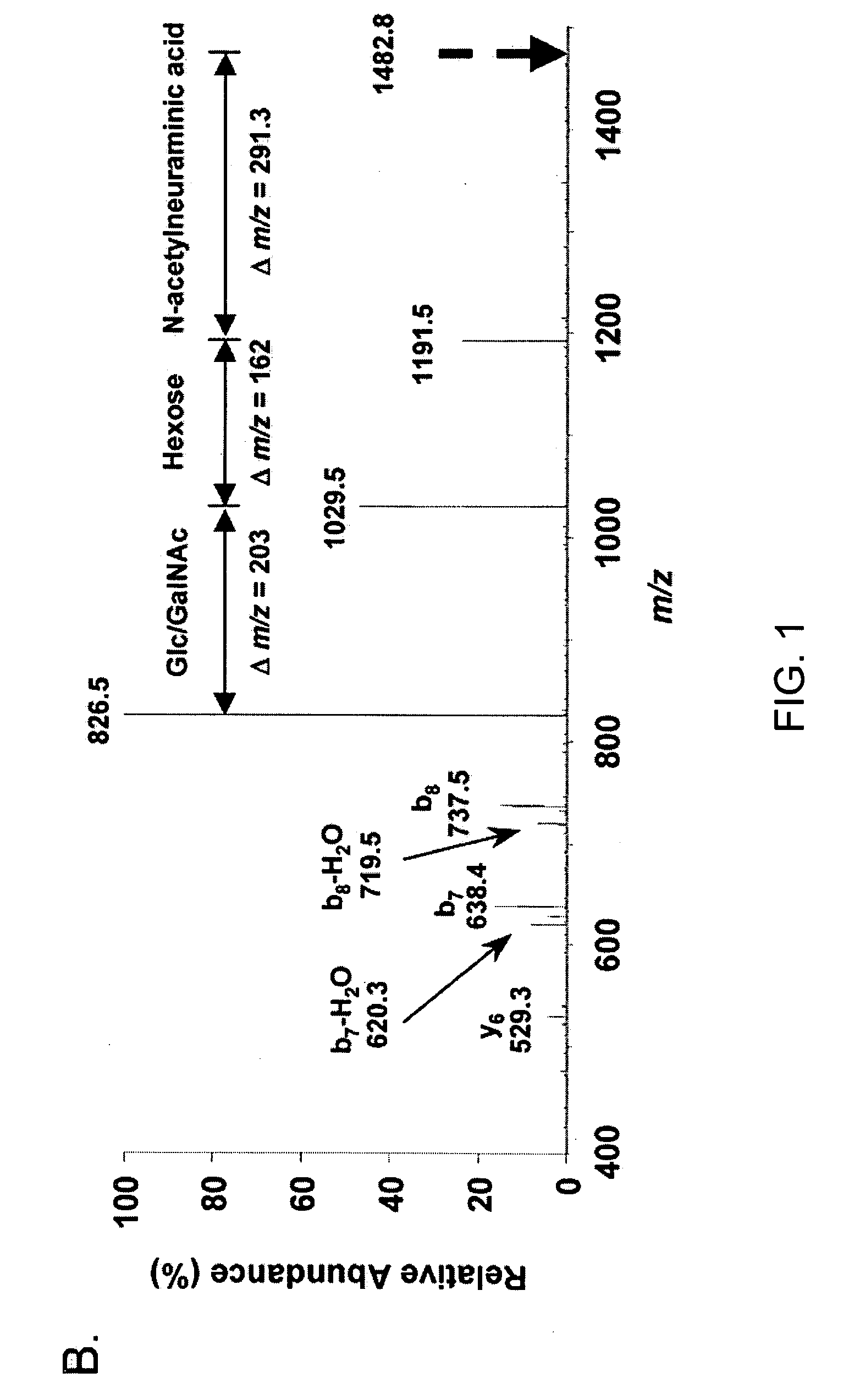
[0222] Microcapillary reversed-phase liquid chromatography was used to obtain extremely pure preparations of APF peptide for mass spectrometry. Analysis of three preparations of HPLC-purified APF using the microcapillary technique indicated the presence of three peptide peaks in approximately equal proportions in each preparation, only one of which had antiproliferative activity against primary bladder epithelial cells in vitro. Ion trap mass spectro...
example 3
Identification of Sugar Moieties by Lectin Binding Analysis
[0235] In specific aspects of the invention, an APF molecule is identified and / or characterized by any suitable means in the art. In one specific embodiment, lectin binding analysis is utilized to identify sugar moieties. Other methods for sugar identification including, but not limited to, chemical degradation, NMR spectroscopy, mass spectrometry, and antibody binding can also be used to identify sugar moieties.
[0236] To determine the identity and linkage of the hexose and hexosamine moieties, HPLC-purified APF was incubated in its native state with various agarose-conjugated lectins and the eluates tested for antiproliferative activity (Table 2).
TABLE 2Lectin Binding AnalysisAPF BindingNeuraminidase-LectinReported SpecificityNativetreatedWheat germ agglutininsialic acid; terminal+NDGlcNAcTritrichomonassialic acid+NDPeanut agglutininGalβ1-3GalNAc;−+GalactoseConcanavalin AMannose, Glucose−−Lentil lectinMannose, Glucose;−...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Polarity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com