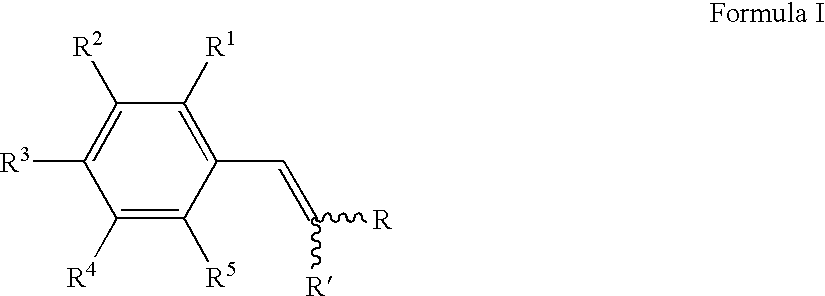
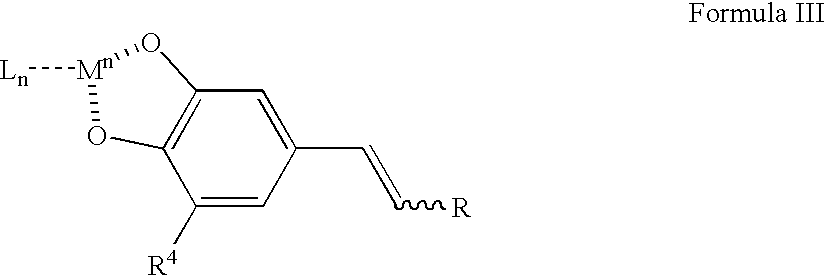
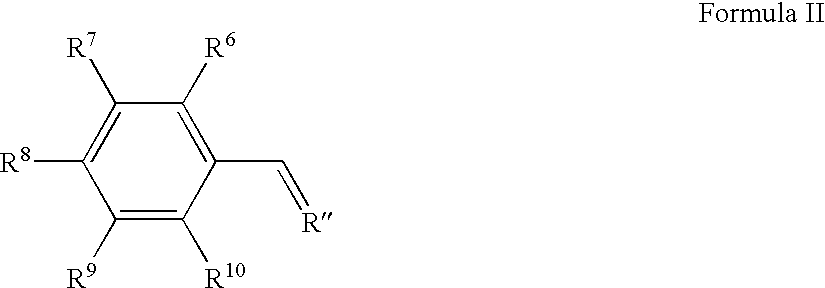Cytoprotective compounds, pharmaceutical and cosmetic formulations, and methods
a technology of cytoprotective compounds and compounds, applied in the field of cytoprotective compounds, can solve the problems of not always being able to distinguish between "preventing" and "suppressing", exposure to sunlight can pose a number of skin health hazards, and the erythema is a long-term hazard of prolonged exposure to sunligh
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 4-[2-(3,4-bis-methoxymethoxy-phenyl)-vinyl]-benzoic Acid Ethyl Ester
[1028] 19
[1029] 1A. (4-Methoxycarbonyl-benzyl)-triphenyl-phosphonium bromide. A solution of, methyl 4-(bromomethyl)-benzoate (2.29 g, 10 mmol) and triphenylphosphine (2.62 g, 10 mmol) in toluene (40 mL) was refluxed for 2 hours and then cooled down to room temperature. The precipitate was filtered and dried under vacuum, giving 4.71 g (95%) of white solid. 20
[1030] 1B. 3,4-Bis-methoxymethoxy-benzaldehyde. To a solution of 3,4-dihydroxbenzaldehyde (2.76 g, 20 mmol, Adrich) in anhydrous N,N-dimethylformamide (DMF, 50 mL, Adrich) was added sodium hydride (1.76 g, 44 mmol, 2.2 eq, 60% in mineral oil, Adrich) portionwise under nitrogen at 0.degree. C. The reaction mixture was stirred at room temperature for 30 min. To the dark blue mixture was added chloromethyl methylether (6.1 mL, 6.44 g, 80 mmol, 4.0 eq, tech, Adrich) dropwise with an ice-water bath and anhydrous potassium carbonate (8.29 g, 60 mmol, 3....
example 2
Preparation of 4-[2-(3,4-Dihydroxy-phenyl)-vinyl]-benzoic Acid Ethyl Ester
[1062] 21
[1063] 2A. To a solution of 4-[2-(3,4-bis-methoxymethoxy-phenyl)-vinyl]-be-nzoic acid ethyl ester (1.0 mmol) of 3 in MeOH (100 mL) at room temperature was added slowly 15 drops of a concentrated HCl solution. The resulting solution was stirred for 12 h. The solvent was then evaporated under reduced pressure and the residue was chromatographed (hexane: EtOAc=1:1) to afford the product as a yellow solid (yield 90%). NMR indicated the purified product contains cis / trans isomers and both ethyl and methyl esters. .sup.1H-NMR (300 MHz, CD.sub.3OD) .delta. 7.98 (d, J=8.4 Hz, 1.82H), 7.87(d, J=8.4 Hz, 0.18H), 7.54 (d, J=8.4 Hz, 1.82H), 7.37 (d, J=8.4 Hz, 0.18H), 7.16-6.92 (m, 4H), 6.81 (m, 1H), 4.41-4.35 (m, 1.9H), 3.92 (m, 0.5H), 1.41 (m, 2.8H).
[1064] 2B. Other Compounds of Formula I Similarly, by following the procedures of Example 2A, there are obtained:
[1065] 4-[2-(3,4-Bis-{2-[2-(2-methoxy-ethoxy)-ethoxy]...
example 3
Preparation of 4-[2-(3,4-Dimethoxymethoxy-phenyl)-vinyl]-nitrobenzene
[1070] 22
[1071] 3A. To a solution of (4-nitrobenzyl)-triphenyl-phosphonium bromide (725 mg, 1.51 mmol, prepared in 97% yield in a similar way as described in Example 1) and 3,4-bis-methoxymethoxy-benzaldehyde (299 mg, 1.38 mmol) in ethanol (20 mL) was added lithium ethoxide (1.45 mL, 1.45 mmol, 1 M in tetrahydrofuran) at room temperature over 4 hours. The resulting solution was stirred at room temperature overnight. The solvent was removed under reduced pressure and the residues were chromatographed (silica gel, methylene chloride), affording an orange-red product (214 mg, 41%). .sup.1H-NMR (300 Hz, D.sub.3CCl) .delta. (ppm) 8.25-8.05 (m, 2H), 7.65-6.95 (m, 5H), 6.85-6.50 (m, 2H), 5.31 (s, CH.sub.2, 40%), 5.28 (s, CH.sub.2, 40%), 5.23 (s, CH.sub.2, 60%), 5.05 (s, CH2, 60%), 3.56 (s, CH.sub.3, 40%), 3.53 (s, CH.sub.3, 40%), 3.51 (s, CH.sub.3, 60%), 3.38 (s, CH.sub.3, 60%).
[1072] B. Other Compounds of Formula I Simil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Stress optical coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com