Preparation and application of low-toxicity Kdo2-monophosphoryl lipid A containing five fatty acid chains
A monophosphoric acid lipid and fatty acid chain technology, applied in the field of bioengineering, can solve the problems of cumbersome extraction process, no extraction and purification method, large molecular weight, etc., and achieve the effect of simple extraction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
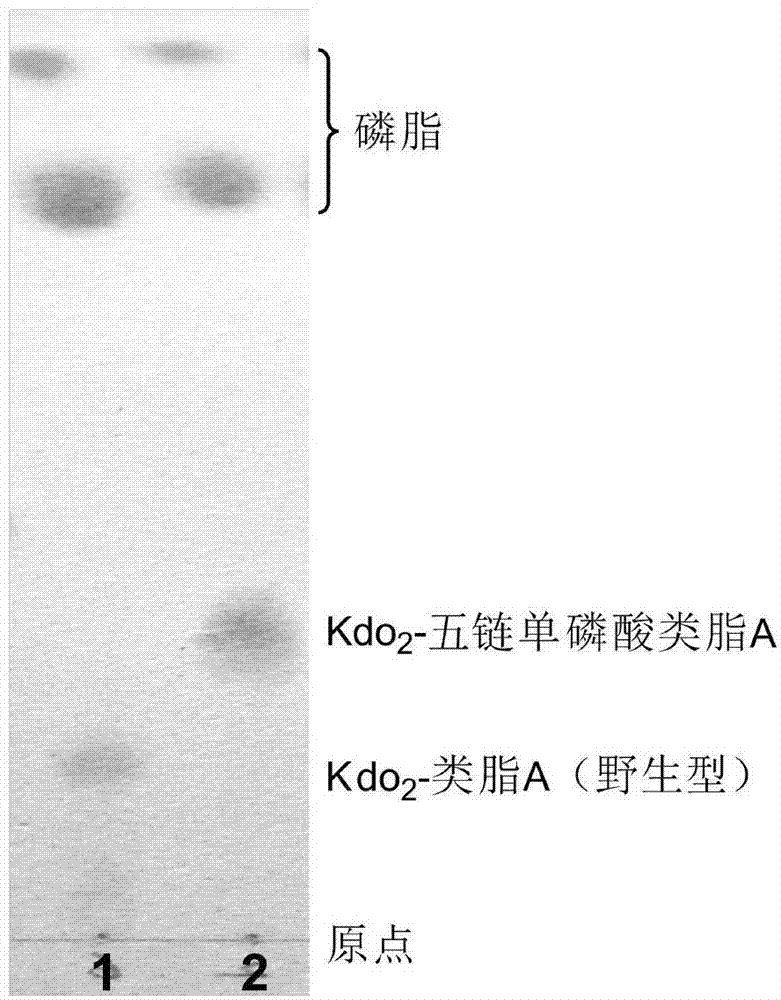
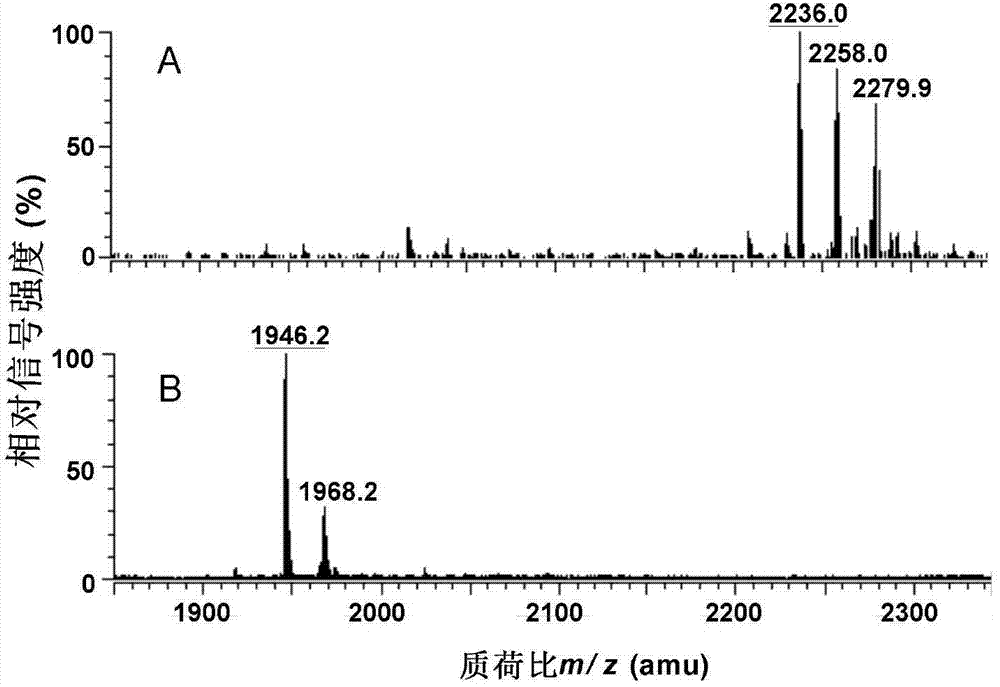
[0027] The Kdo produced by the genetic engineering bacteria BW002 of embodiment 1 2 - Extraction and structural analysis of lipid A
[0028] LPS produced by W3110 and containing long sugar chains cannot be directly extracted, and its structure cannot be directly analyzed by thin layer chromatography (TLC) and ESI / MS analysis due to its large mass and low solubility in the organic phase. Therefore, we selected Escherichia coli WBB06 (W3110waaCF::tet6), the heptose-deficient mutant of W3110 as a control for structural analysis. WBB06 was confirmed to be able to directly synthesize Kdo with six fatty acid chains and bisphosphoric acid in lipid A part 2 - Lipid A.
[0029] 1. Kdo 2 - Extraction of Lipid A
[0030] Kdo produced by control bacteria WBB06 and genetically engineered bacteria BW001 2 -Lipid A was extracted using a chloroform / methanol / water mixed phase extraction method. The specific operation is as follows: the seed bacteria liquid cultured overnight was 600 =0....
Embodiment 2
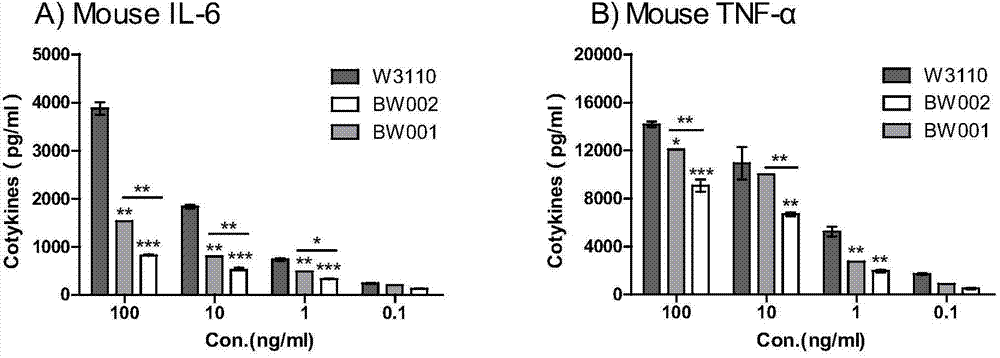
[0035] The five-chain Kdo produced by the genetic engineering bacteria BW002 of embodiment 2 2 - Toxicity analysis of monophospholipid A
[0036] Kdo extracted directly 2 -Lipid A sample is mixed with various membrane phospholipids, so it needs to be purified to remove phospholipid interference before performing cytotoxicity analysis. Kdo 2 - The purification of lipid A is mainly accomplished by gradient elution on a DEAE fiber column. In the cell stimulation experiment, the purified sample of lipopolysaccharide (LPS) from the wild-type strain W3110 was used as a positive control, and PBS was used as a hard control; an attenuated strain produced by genetically engineered bacteria BW001 (E.coli W3110△waaCF△lacIlacZ::FnlpxE) was also introduced. , monophosphate six fatty acid chains Kdo 2 - Lipid A for comparison.
[0037] 1. Kdo 2 - DEAE fiber column purification of lipid A
[0038] The genetically engineered bacteria BW002 and the control engineered bacteria BW001 were ...
Embodiment 3
[0045] Embodiment 3 five-chain Kdo with low toxicity 2 -Analysis of hydrophobicity and self-aggregation ability of genetically engineered bacteria BW002 producing monophosphate lipid A
[0046] 1. Comparison of hydrophobicity between genetically engineered bacteria BW002 and wild-type Escherichia coli W3110
[0047] The method for measuring the hydrophobicity of the bacterial strain is as follows: overnight cultured bacterial liquid is centrifuged (7000g, 10min) to collect the bacterial cells; the bacterial cells are washed twice with PBS7.4; then the bacterial cells are suspended with PBS7.4 to make the OD 600 =1.0 (marked as A 0 , keep 3 decimal places); Finally, mix 2mL bacterial suspension with 800μL xylene, vortex for 2min, and measure the OD of the aqueous phase after standing at room temperature for 1h 600 (recorded as A, keep 3 decimal places), calculate cell surface hydrophobicity: H%=(A 0 -A) / A 0 *100.
[0048] Since LPS is the main macromolecule on the surface ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydrophobicity | aaaaa | aaaaa |
| hydrophobicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com