Cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant co-loading antigen, mpla and imq, preparation method and application
A vaccine adjuvant and cation technology, which is applied in the field of cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant and preparation, can solve the problems of rapid drug leakage, low biocompatibility, and low drug load.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The invention provides a preparation method of an antigen-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, comprising the steps of:
[0035] S1: Dissolve the amphiphilic triblock copolymer PCL-b-PEG-b-PCL and the cationic phospholipid DOTAP in an organic solvent, then remove the organic solvent by rotary evaporation, and form a uniform film on the bottle wall, blow with nitrogen Dry the residual solvent, then put it in a vacuum drying oven, and dry it in vacuum for 12-24h; wherein, the molecular weight of the amphiphilic triblock copolymer PCL-b-PEG-b-PCL is 10000-24000, preferably 16000, wherein PEG The mass percentage of the hydrophilic segment is greater than 45%, the mass ratio of the amphiphilic triblock copolymer PCL-b-PEG-b-PCL to the cationic phospholipid DOTAP is 20 mg: (0.5-2) mg; the organic solvent is selected from acetonitrile, A mixture of one or more of dichloromethane and chloroform;
[0036] Preferably, it also includes the st...
Embodiment 1
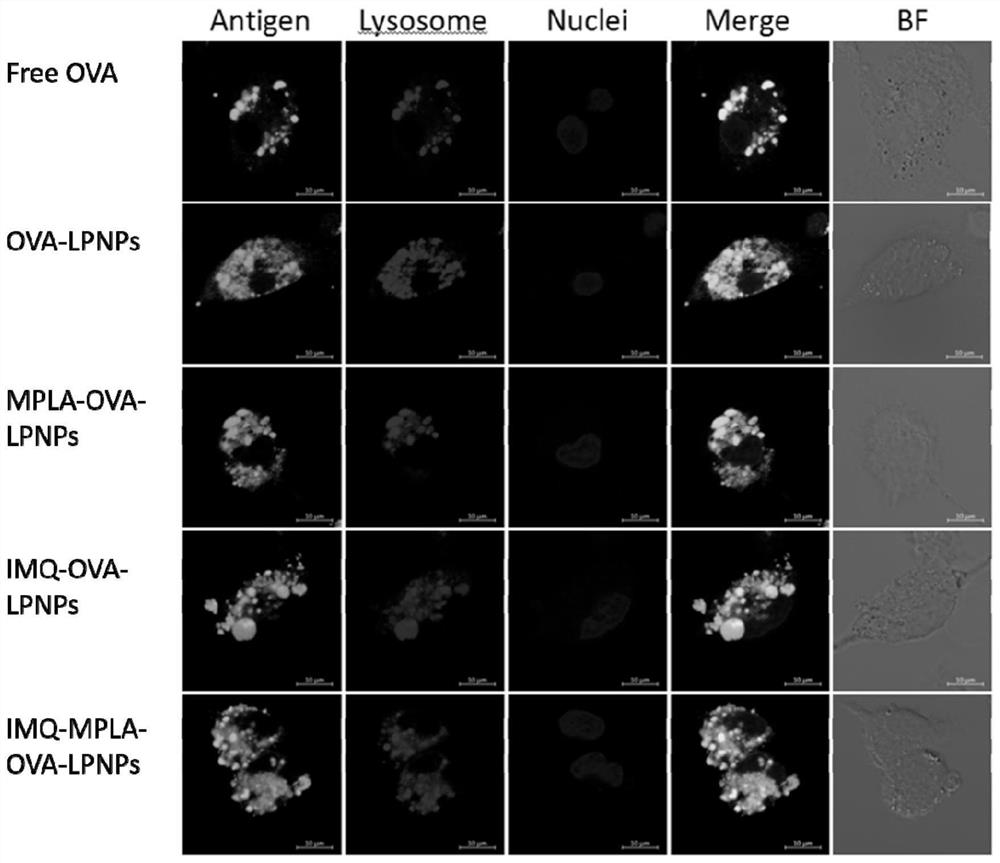
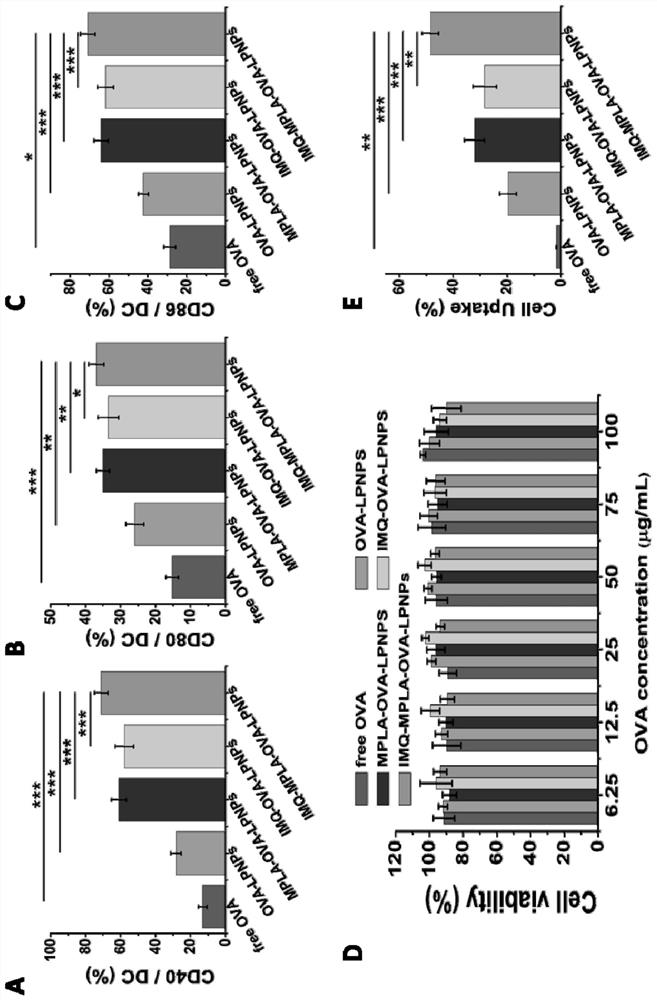
[0041] This embodiment provides a cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant loaded with antigen OVA. The preparation method comprises steps:
[0042] S1: Dissolve 20mg of amphiphilic triblock copolymer PCL-b-PEG-b-PCL and 1mg of cationic phospholipid DOTAP in dichloromethane, then remove the organic solvent by rotary evaporation, and form a uniform film on the bottle wall, Dry the residual solvent with nitrogen, then put it in a vacuum drying oven, and dry it in vacuum for 12-24 hours; among them, the molecular weight of the amphiphilic triblock copolymer PCL-b-PEG-b-PCL is 16000, and the PEG hydrophilic chain Segment mass percentage greater than 45%.
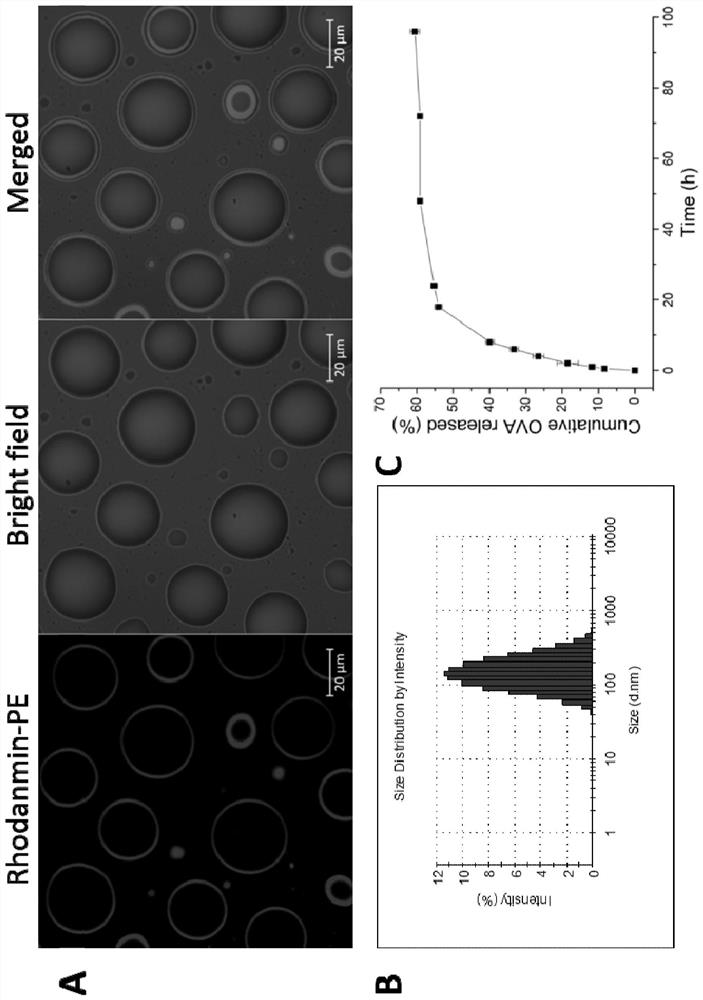
[0043] S2: Add 10 mL of double-distilled water to the dried product, hydrate at 65°C for 5 hours, oscillate and mix evenly, and then sonicate for 10 minutes in an ice bath to form a stable emulsion, filter the stable emulsion with a 0.45 μm filter membrane, collect the filtrate, and obtain Cationic pho...
Embodiment 2
[0047] This embodiment provides a cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant loaded with TLR7 agonist IMQ and antigen OVA. The preparation method includes the steps:
[0048] S1: 20 mg amphiphilic triblock copolymer PCL-b-PEG-b-PCL, 1 mg cationic phospholipid DOTAP, and 100 μg TLR7 agonist IMQ were dissolved in dichloromethane, and then the organic solvent was removed by rotary evaporation, forming a layer a uniform film, dry the residual solvent with nitrogen, put it in a vacuum drying oven, and dry it in vacuum for 12 hours; wherein, the molecular weight of the amphiphilic triblock copolymer PCL-b-PEG-b-PCL is 16000, of which PEG The mass percentage of the hydrophilic segment is greater than 45%.
[0049] S2: Add 10 mL of double-distilled water to the dried product, hydrate at 65°C for 5 hours, oscillate and mix evenly, and then sonicate for 10 minutes in an ice bath to form a stable emulsion, filter the stable emulsion with a 0.45 μm filter membrane,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com