Methods for the production of 3-o-deactivated-4'-monophosphoryl lipid a (3d-mla)
a technology of monophosphoryl lipid and production method, which is applied in the direction of antibody medical ingredients, drug compositions, immunological disorders, etc., to achieve the effect of simple and inexpensive steps and reduced phospholipid conten
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Methods
[0063] A. Media Preparation
[0064] Cell growth was conducted in M9 medium, which is prepared by combining sterile solutions of inorganic salts, casamino acids, and dextrose. The M9 salt solution is typically prepared in the fermentor and contains the following salts: 2.0 g / L NaCl, 0.2 g / L MgSO4.7H2O, 3.0 g / L KH2PO4, 6.0 g / L Na2HPO4, and 1.0 g / L NH4Cl. Sterile solutions of 20% (w / v) casamino acids (20 mL / L) and 50% (w / v) dextrose (32 mL / L) and then added aseptically to the fermentor to yield the completed medium.
[0065] B. Seed Growth
[0066] Typically, a sterile 250 mL Erlenmeyer flask was charged with 50 mL sterile M9 medium. A seed vial of Salmonella minnesota R595 (ca. 108 cfu) was thawed and added to the flask, which was then stoppered with a gauze plug. The culture was incubated at 37° C. for 6-8 h, until robust growth is evident.
[0067] C. Cell Growth
[0068] Cultures of Salmonella minnesota R595 were grown in a BioFlo III fermentor (New Brunswick Scientific, Inc...
example 2
Analytical Methods
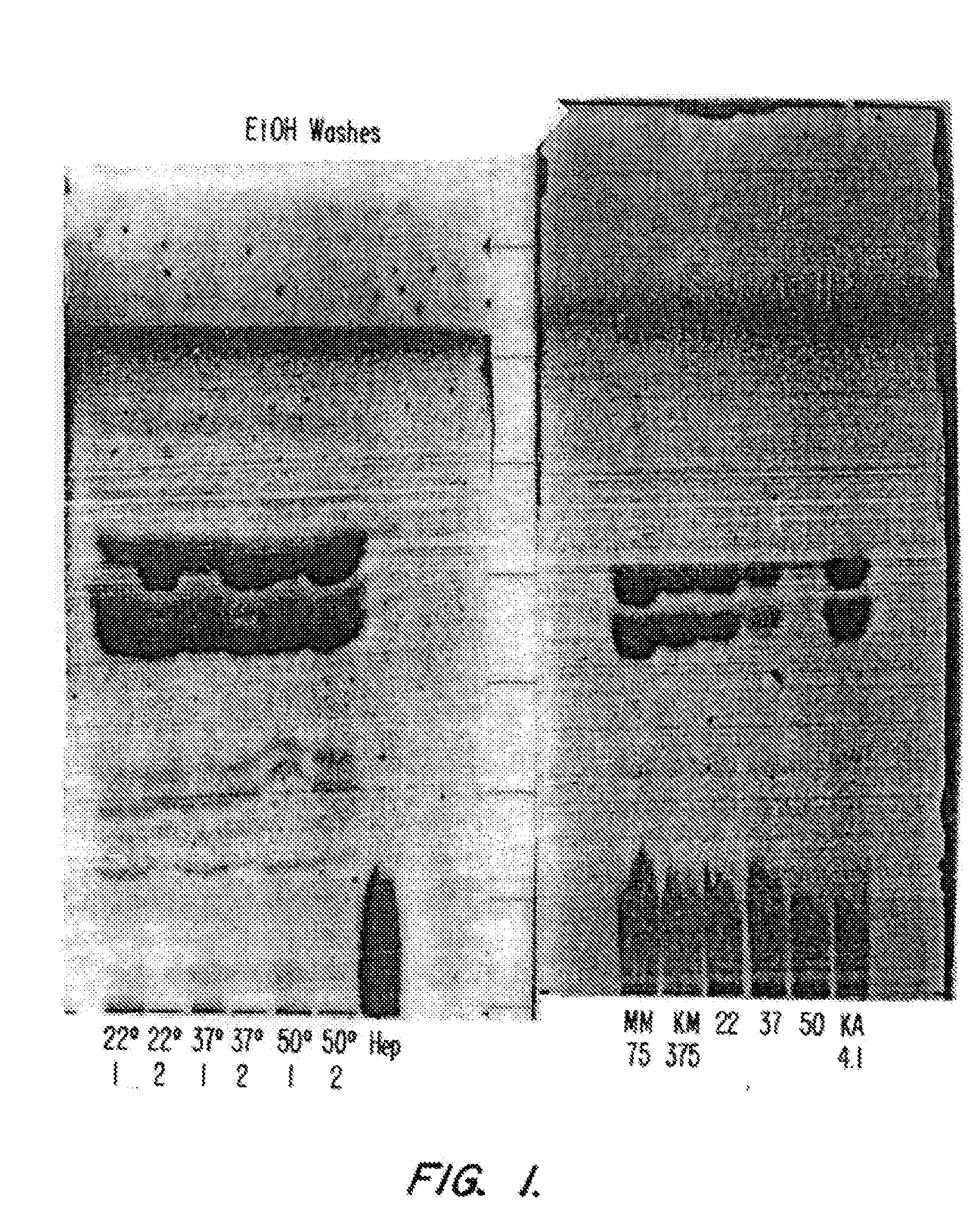
[0075] A. Thin Layer Chromatography (TLC) of MLA and Related Samples
[0076] All TLC analyses were carried out using 5×10 cm plates coated with Silica Gel 60 (E Merck). Samples were generally applied to the TLC plates as 10 mg / mL solutions in chloroform:methanol 4:1 (v / v), with 3 μL solution (30 μg sample) applied in small spots to a 5 mm line using a capillary pipette. Plates were developed with a solvent system comprising chloroform / methanol / water / ammonium hydroxide 50:31:6:2 (v / v). Bands on the developed plates were visualized by spraying with a solution of 10% (w / v) phosphomolybdic acid in ethanol followed by charring at 150-160° C. In some cases, relative intensities of spots were quantified by scanning densitometry with a Shimadzu CS9000U Dual Wavelength Flying Spot Scanner (Shimadzu Corp.), using a scanning wavelength of 520 nm.
[0077] B. Analysis of MLA / 3D-MLA by High Performance Liquid Chromatography (HPLC)
[0078] Samples to be analyzed were first converte...
example 3
Comparison of Congener Composition of MLA / 3D-MLA from Cultures Harvested at Different Times
[0083] A series of fermentor runs was conducted with the following parameters: 2.0 L M9 medium (initial pH 6.84-6.87), 2 Lpm air flow, stirring at 50 rpm, 37° C., no pH control. Cultures were monitored by measuring optical density at 590 nm and were stopped when the desired growth stage was attained. Cells were processed and extracted as described above to yield LPS samples, which were then hydrolyzed to MLA and 3D-MLA and analyzed by HPLC (see Examples 1 and 2). Results are summarized in Table 1.
TABLE 1Congener composition of MLA and 3D-MLA from cells harvested at different ages.MLA3D-MLA3-O-3-O-Culture ageTime indeacylateddeacylatedRunDescriptionat harveststationary phasehexaacylheptaacylhexaacylALate exponential6.75 h N / A12.4%12.2%9.9%BEarly stationary9.5 h˜0.5 h9.2%6.8%9.2%phaseCLate stationary 15 h ˜6 h19.5%13.2%21.5%phase
[0084] The data show that cultures of S. minnesota R595 alter t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mol % | aaaaa | aaaaa |
| mol % | aaaaa | aaaaa |
| mol % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com