A vaccine composition comprising an immunogenic protein and combination adjuvants for use in eliciting antigen-specific t-cell responses
A technology of immunogenicity and composition, applied in the field of vaccine preparations with combined adjuvants, which can solve the problems of inability to induce protective immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
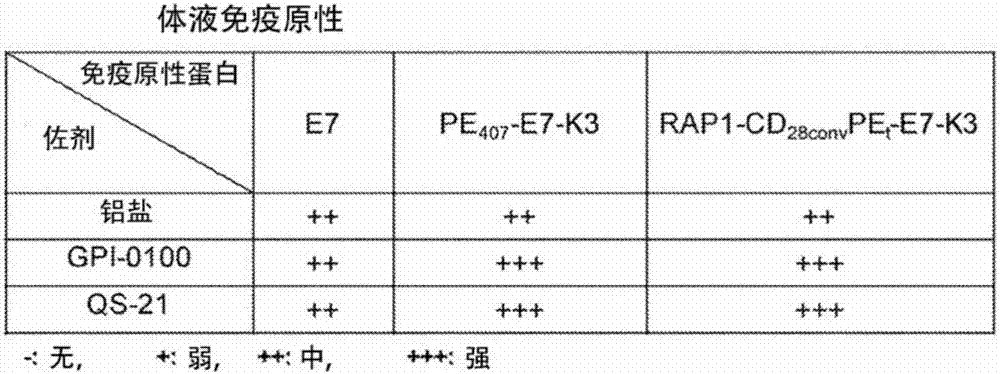
[0131] The following provides exemplary instruments, devices, methods and related results according to the embodiments of the present invention, but does not constitute a limitation to the scope of the present invention. It should be understood that headings or subtitles may be included in the examples for ease of reading, which should not limit the scope of the present invention. In addition, specific theories are presented and disclosed below, however, no matter whether they are right or wrong, any specific theory or action plan should not limit the scope of the present invention as long as the practice of the present invention is not affected.
[0132] Immunogenic protein preparation:
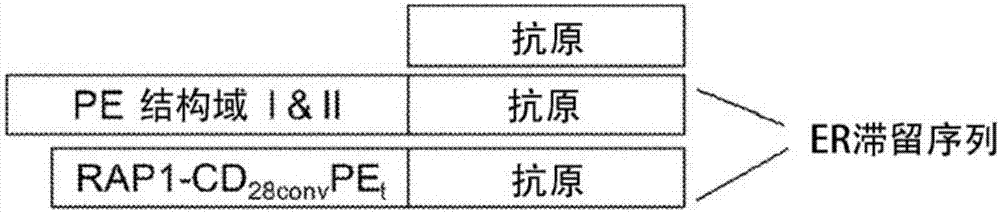
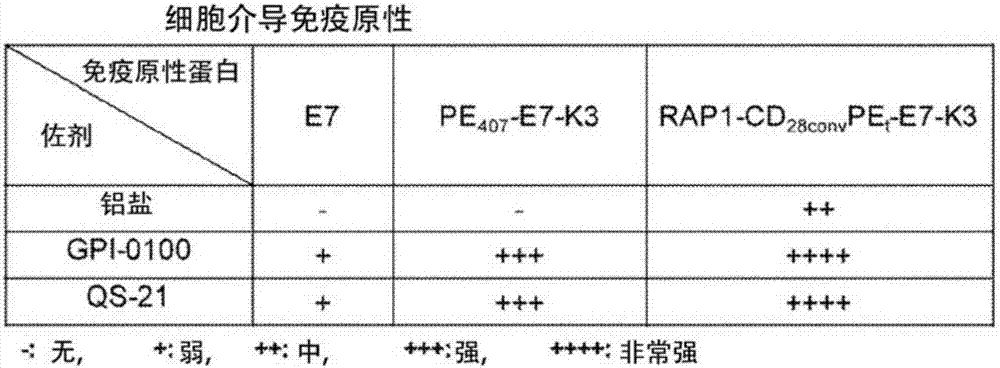
[0133] Immunogenic proteins were expressed in E. coli expression system. It may be antigen alone, or an antigen and ER retention signal (K3) fused to P. aeruginosa exotoxin A domains I and II (i.e., PE 407 ) to the C-terminus to generate PE 407 -(antigen)-K3 fusion protein, or fusion to R...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com