Acquisition method and application of individualized tumor neoantigen specific CD8 cells
A CD8 cell and acquisition method technology, which is applied in the field of acquisition of individualized tumor neoantigen-specific CD8 cells, can solve the problems of high chance of contamination, time-consuming, short survival period of DC cells, etc., and achieve the effect of controlling tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for obtaining individualized tumor neoantigen-specific CD8 cells, the steps comprising:
[0027] (1) After the patient is injected with tumor neoantigen vaccine, 50 mL of blood is collected;
[0028] (2) Obtain 3-5×10 peripheral blood mononuclear cells by density gradient centrifugation 8 , use anti-human CD14 antibody magnetic beads to obtain CD14-positive mononuclear cells with a purity >90%, and freeze CD14-negative cells (lymphocytes);
[0029] (3) Mononuclear cells 2×10 6 Cultivate using serum-free GMP CellGro medium, said serum-free GMPCellGro medium contains 1000U / mL granulocyte-macrophage colony growth factor and 1000U / mL recombinant human interleukin-4;
[0030] (4) On the third day, add 2.5ug / mL artificially synthesized monophosphoryl lipid A and 1000 U / mL pharmaceutical grade interferon gamma to the medium, and continue to culture for 24 hours;
[0031] (5) On day 4, mature DC cells were collected, placed in RPMI-GlutaMAX medium containing 10% aut...
Embodiment 2
[0035] The obtained specific CD8 cells were verified by ELISPOT (enzyme-linked immunospot assay) specific detection and RTCA (real-time label-free dynamic cell analysis).
[0036] ELISPOT: Peptide-specific IFN-γEliSpot detection in patients with lung adenocarcinoma after injection of the polypeptide of the USP47 mutant gene (QLVPKEIENV). Control group SPC-A-1 (lung adenocarcinoma cell line), SK-MES-1 (lung squamous cell line).
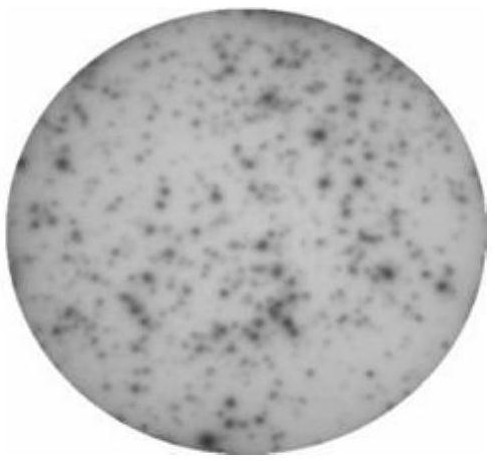
[0037] The results showed that adding SPC-A-1 cells ( figure 1 ) and SK-MES-1 cells ( figure 2 ) whole antigen did not activate CD8 cells to release INF-γ. After adding the polypeptide of the USP47 mutant gene, CD8 cells were activated and released a large amount of INF-γ ( image 3 ).
[0038] RTCA: Through real-time dynamic electrode impedance detection, biological information related to cell physiological functions can be obtained, and the killing effect of specific CD8 cells on tumor cells can be detected.
[0039] The results showed that CD8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com