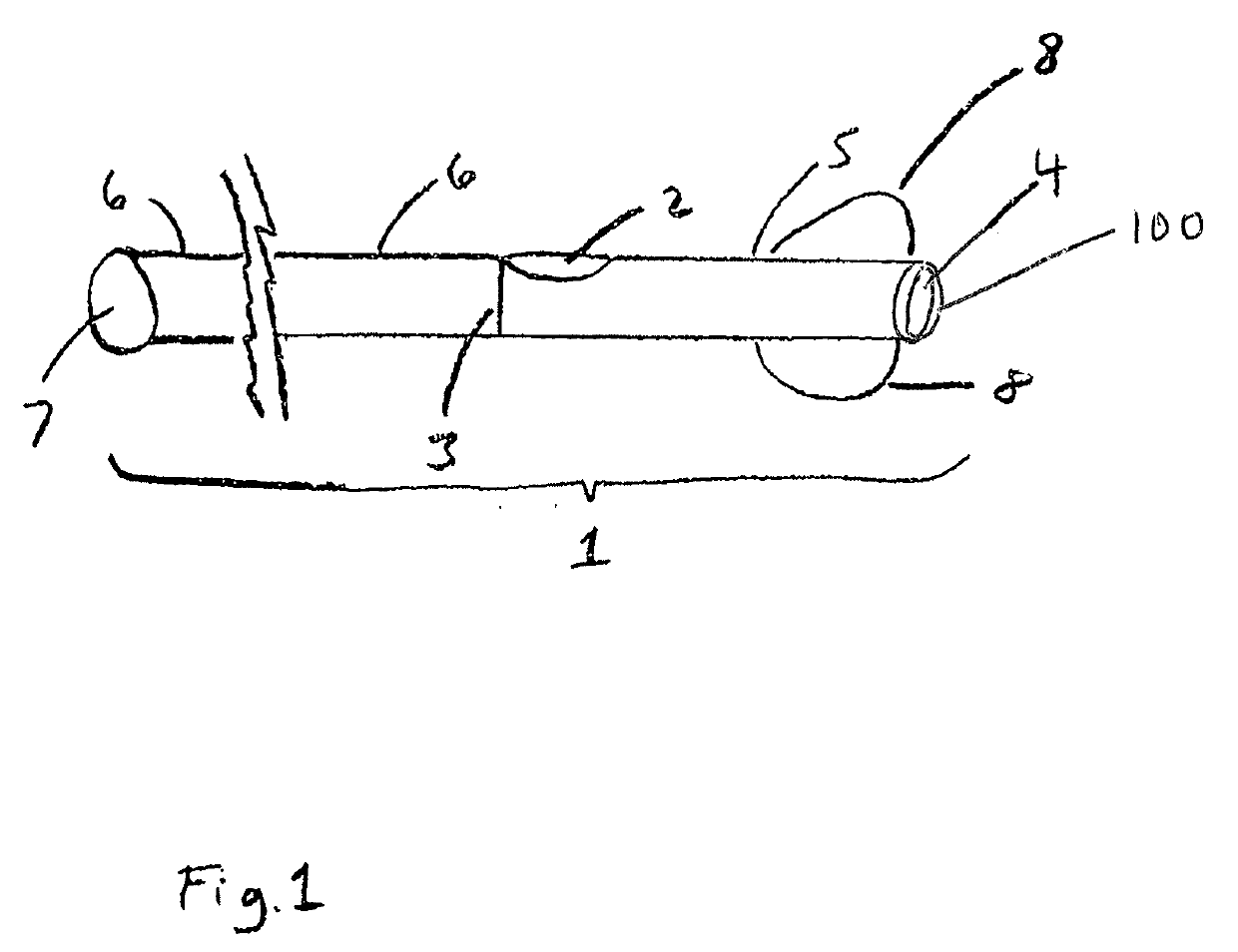
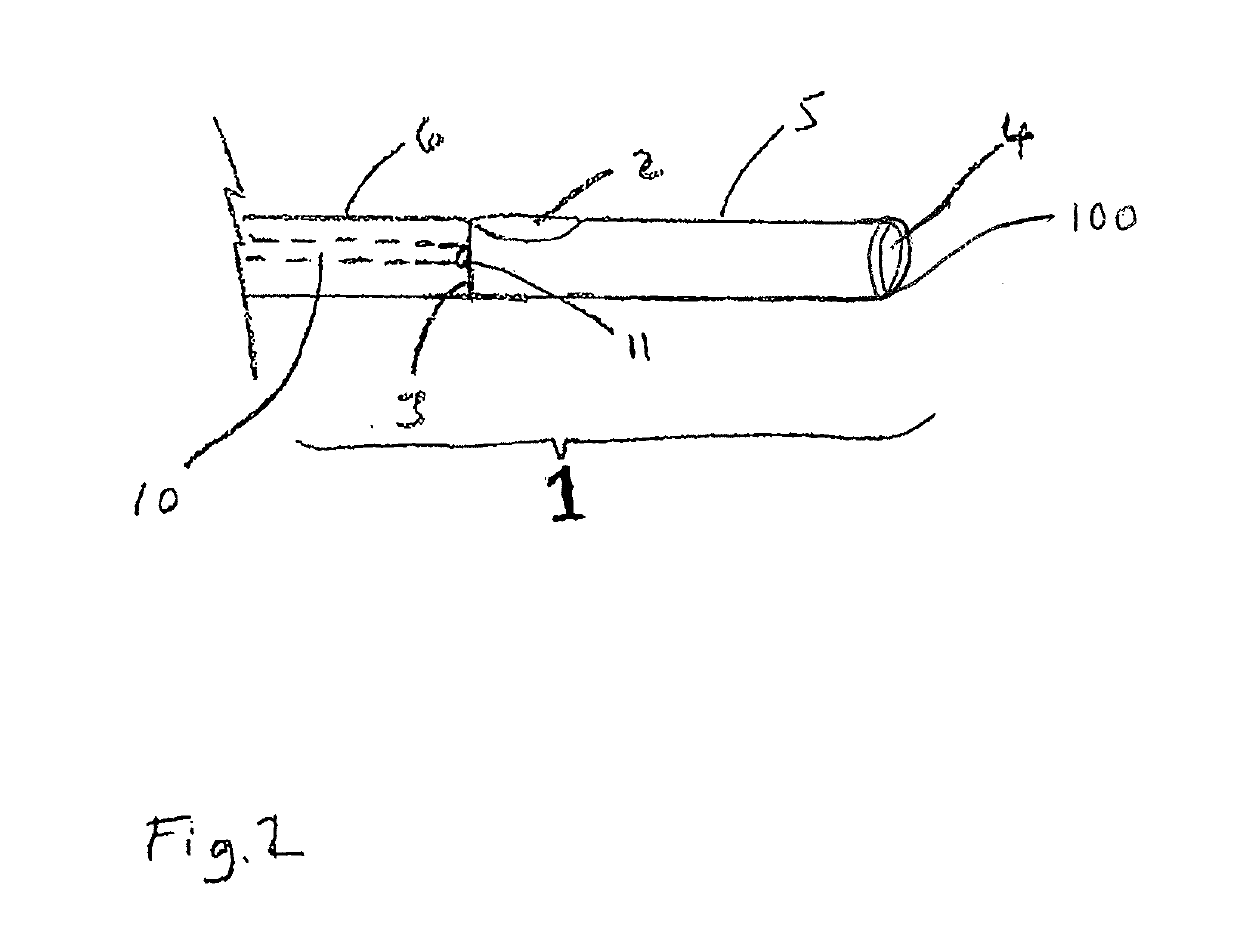
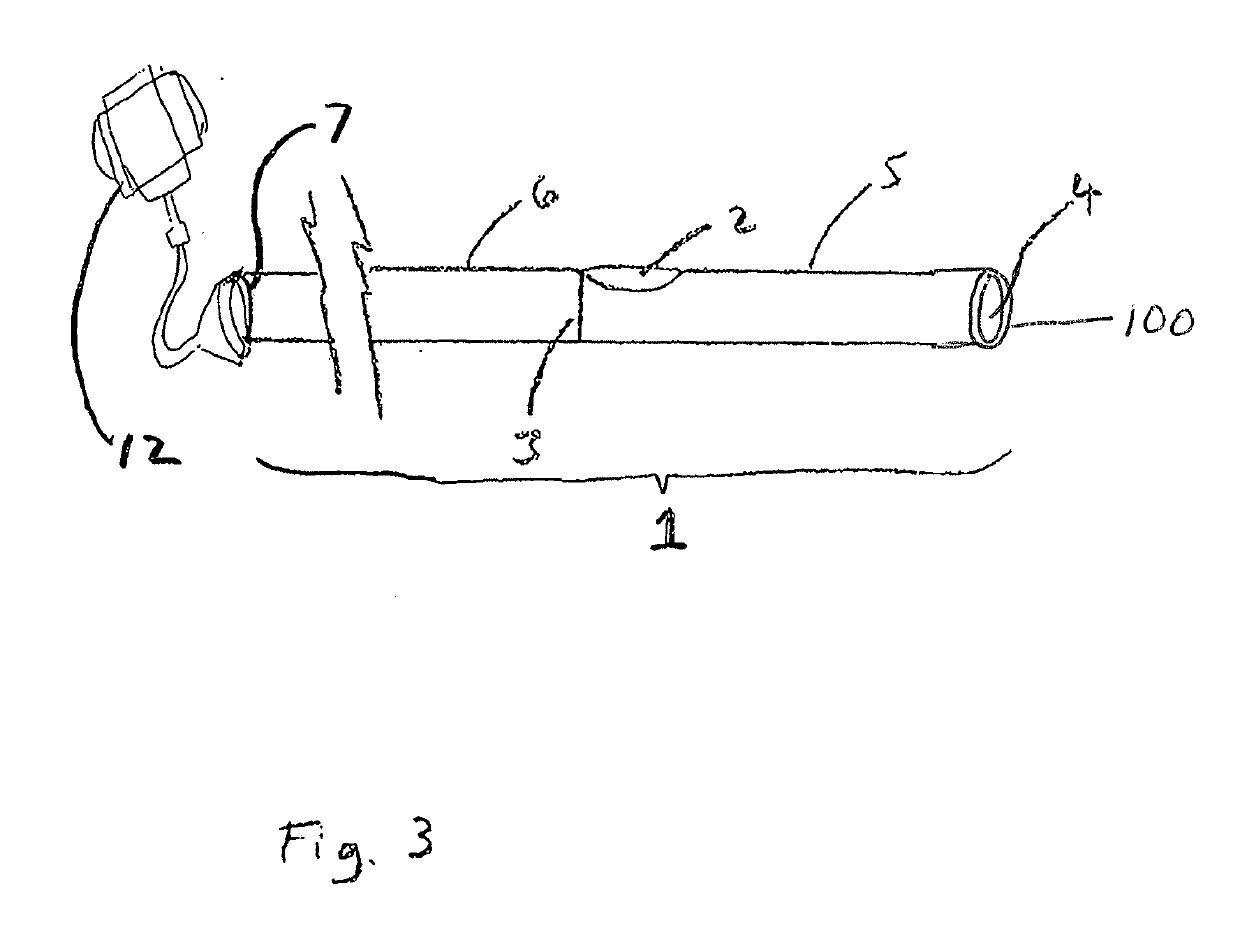
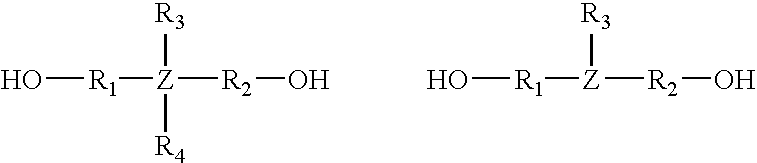Patents
Literature
64results about How to "Low in DO" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nanoparticulate lipase inhibitor formulations
InactiveUS20060246141A1Equal efficacyHigh binding affinityBiocideOrganic active ingredientsChemistryIntestinal Lipase Inhibitor
Owner:ELAN PHRMA INT LTD
Compositions and methods for potentiated activity of biologically active molecules
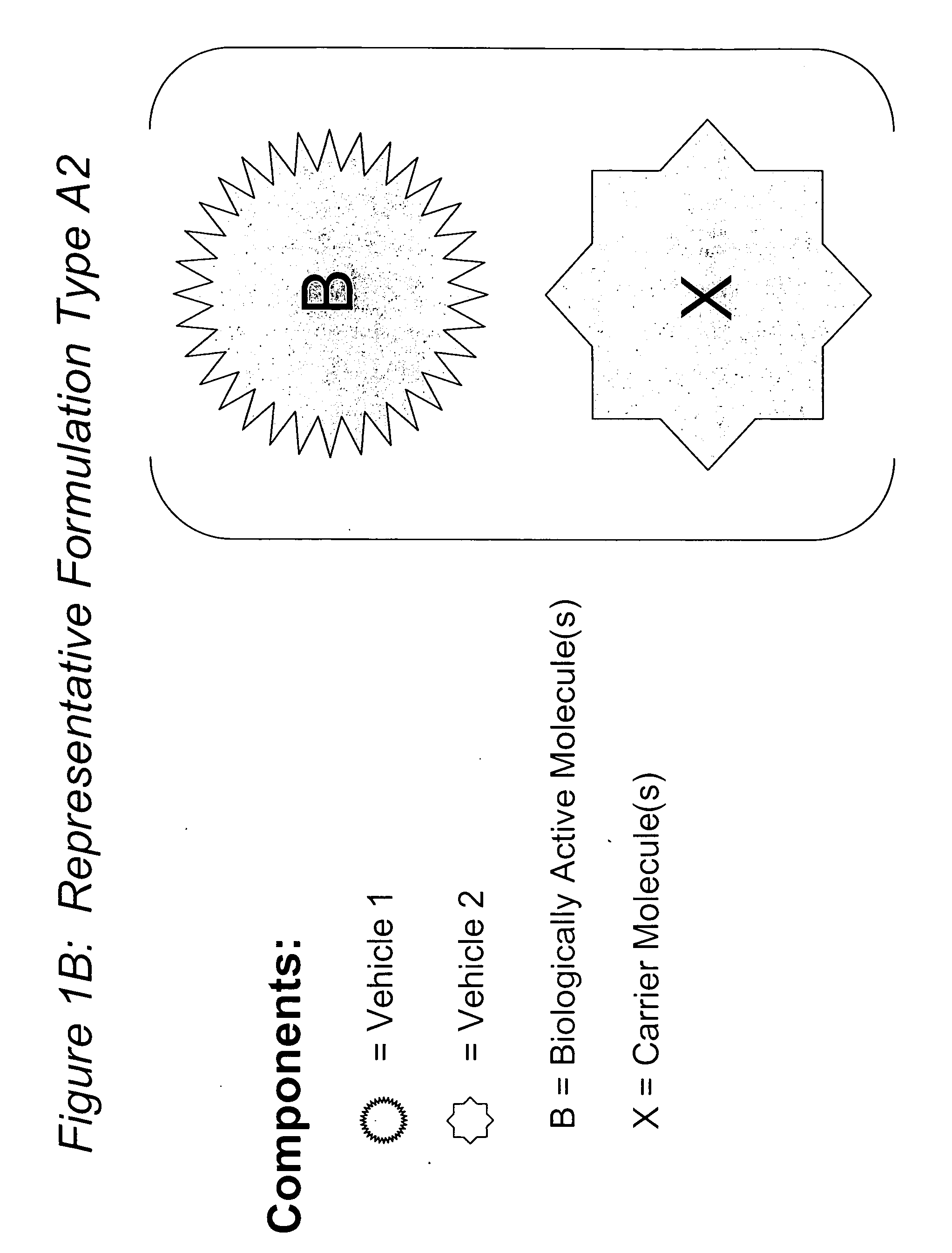
InactiveUS20100015218A1Reduce deliveryLow in DOOrganic active ingredientsMicroencapsulation basedDiseaseLipid formation
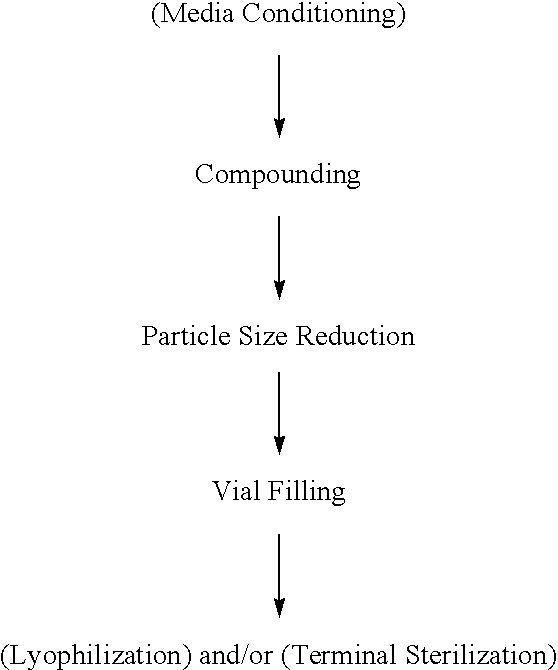
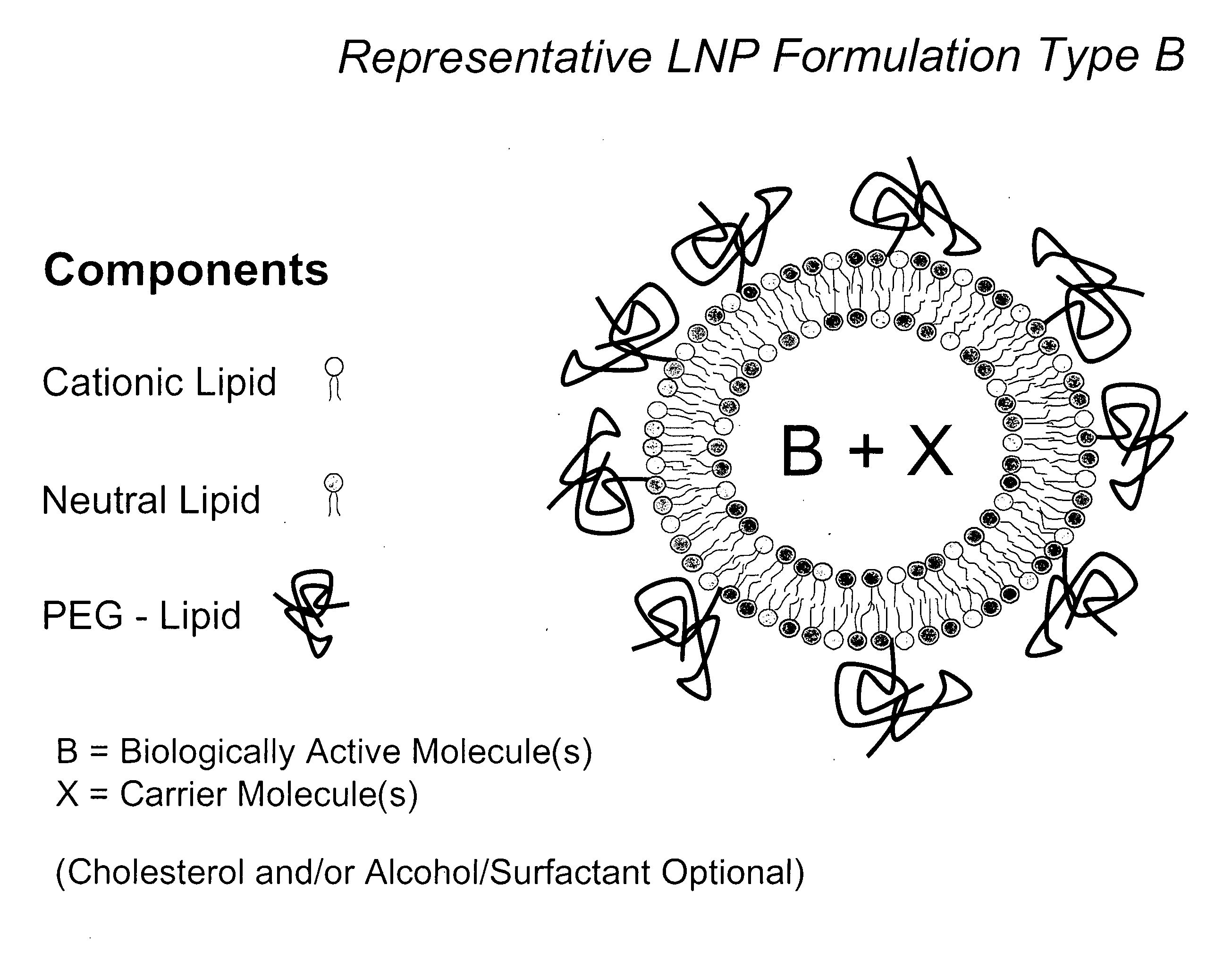
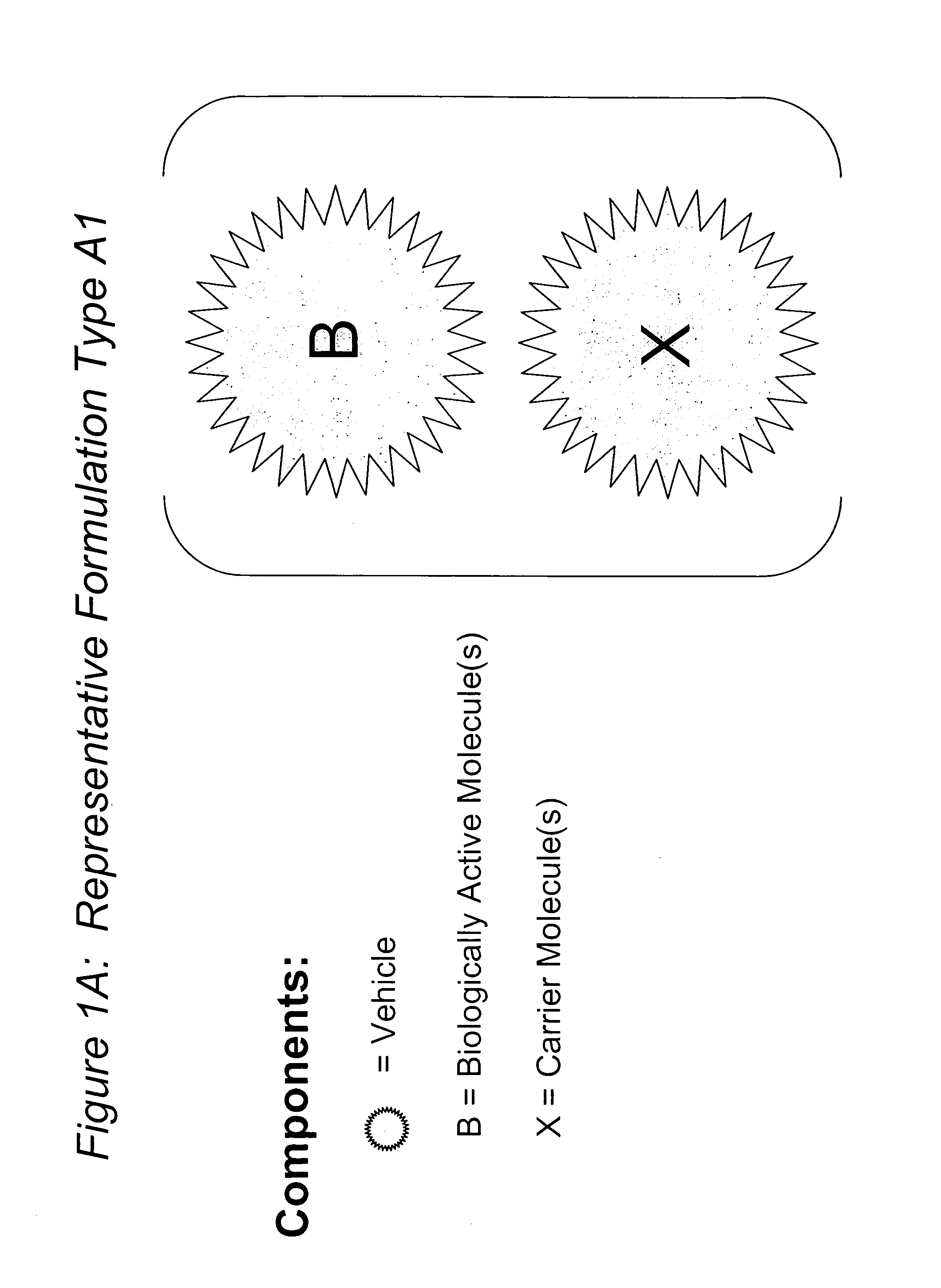
The present invention relates to novel compositions and methods for potentiating the activity of biologically active molecules in conjunction with one or more delivery vehicles and one or more carrier molecules. Specifically, the invention features the use of a carrier molecule in combination with a delivery vehicle and a biologically active molecule of interest to potentiate the activity of the biologically active molecule. The carrier molecule can be biologically inert, inactive, or attenuated; or can alternately be biologically active in the same or different manner than the biologically active molecule of interest. Specifically, the invention features novel particle forming delivery agents including cationic lipids, microparticles, and nanoparticles that are useful for delivering various biologically active molecules to cells in conjunction with a carrier molecule. The invention also features compositions, and methods of use for the study, diagnosis, and treatment of traits, diseases and conditions that respond to the modulation of gene expression and / or activity in a subject or organism that are delivered intracellularly in conjunction with a carrier molecule. In various embodiments, the invention relates to novel cationic lipids, microparticles, nanoparticles and transfection agents that effectively transfect or deliver biologically active molecules, such as antibodies (e.g., monoclonal, chimeric, humanized etc.), cholesterol, hormones, antivirals, peptides, proteins, chemotherapeutics, small molecules, vitamins, co-factors, nucleosides, nucleotides, oligonucleotides, enzymatic nucleic acids, antisense nucleic acids, triplex forming oligonucleotides, 2,5-A chimeras, allozymes, aptamers, decoys and analogs thereof, and small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules, to relevant cells and / or tissues, such as in a subject or organism, in conjunction with one or more carrier molecules. Such novel cationic lipids, microparticles, nanoparticles and transfection agents that are used in conjunction with one or more carrier molecules are useful, for example, in providing compositions to prevent, inhibit, or treat diseases, conditions, or traits in a cell, subject or organism.
Owner:MERCK SHARP & DOHME CORP
Combined Active and Passive Targeting of Biologically Active Agents
InactiveUS20070287680A1Good curative effectLow in DOPolypeptide with localisation/targeting motifOrganic active ingredientsActive agentEfficacy
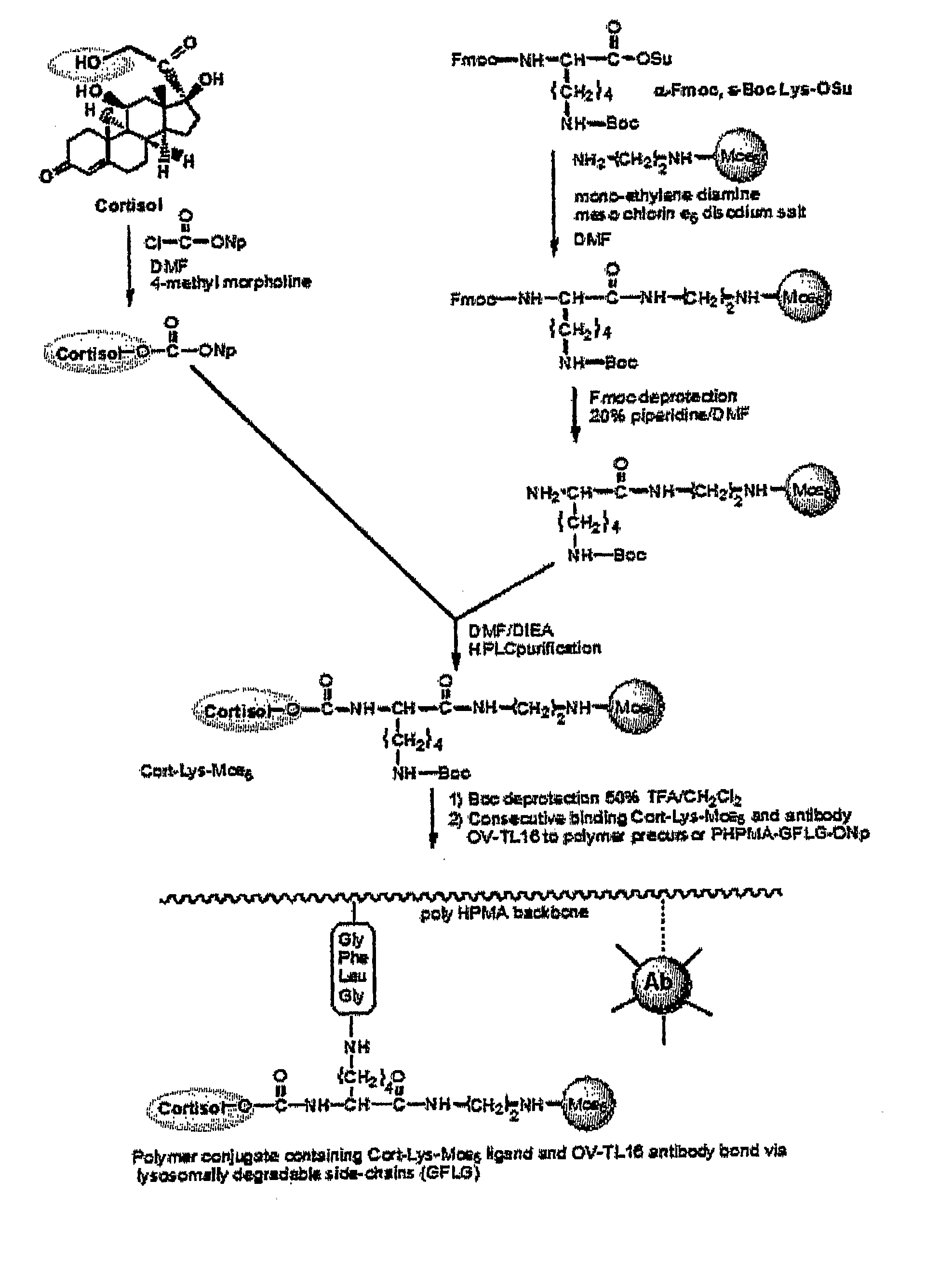
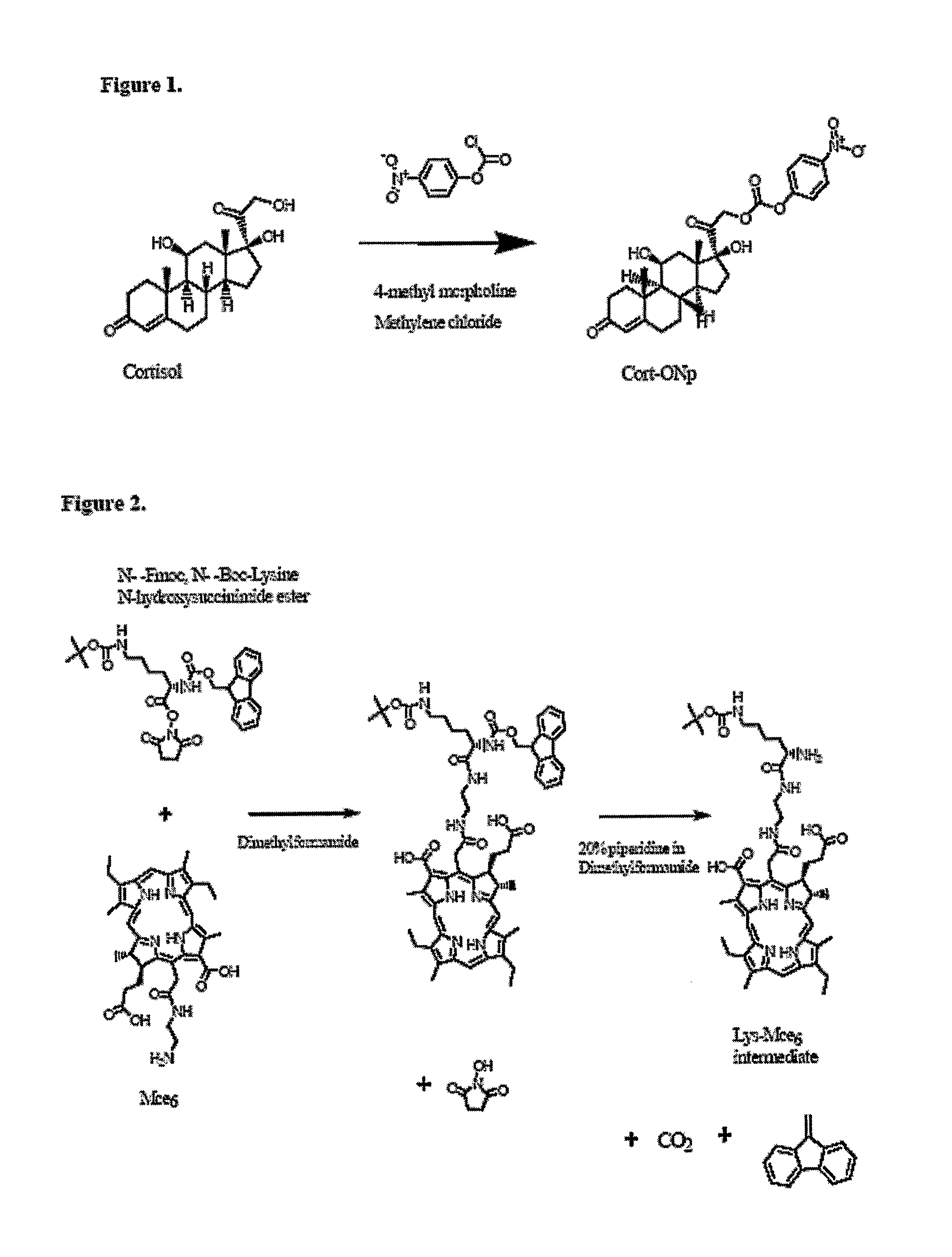
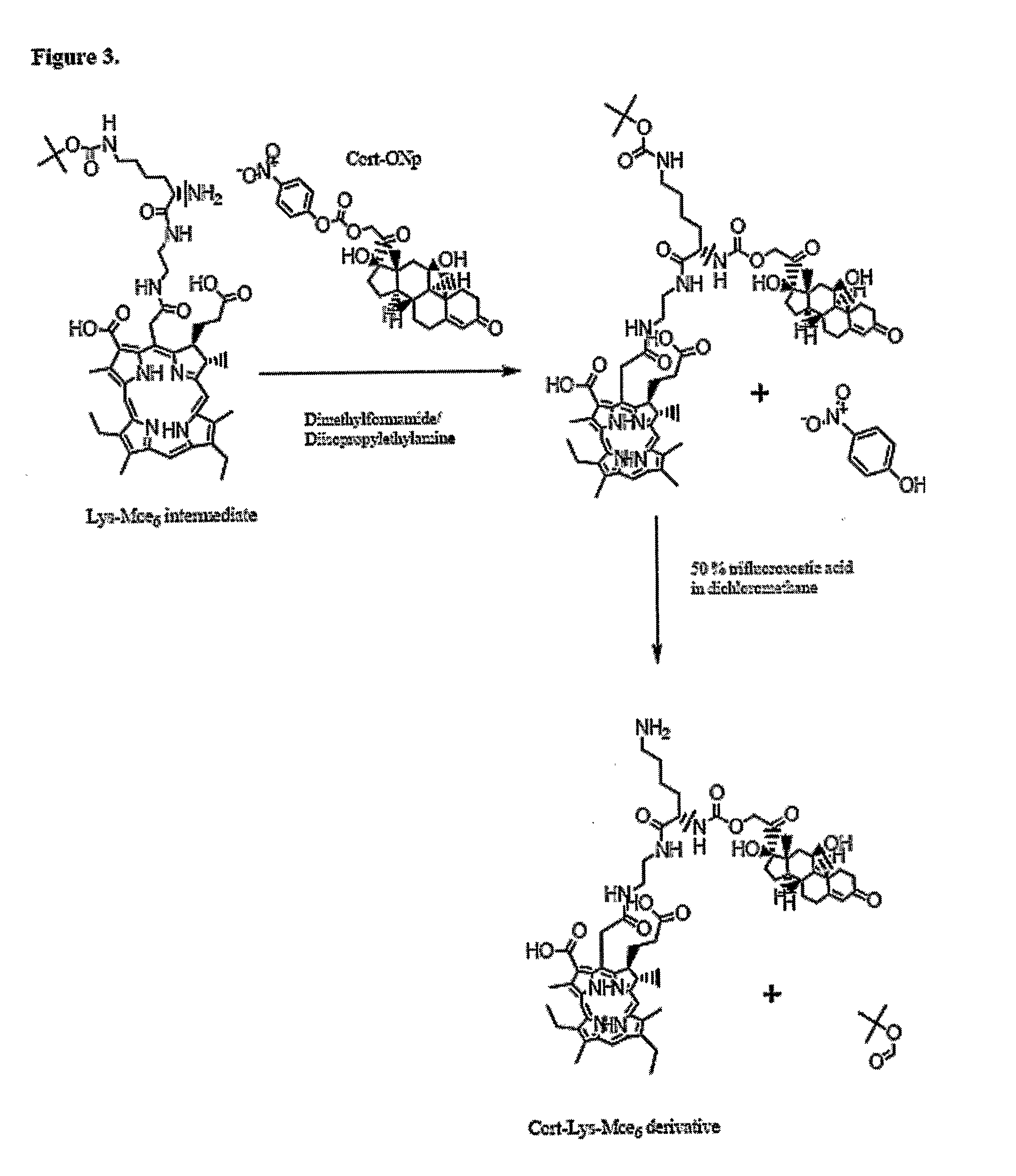
Disclosed is a conjugate comprising a biologically active agent (drug) linked to a subcellular targeting moiety that targets a drug specifically to the nucleus. Targeting is achieved by attaching a steroid hormone (or an analog) to the drug. The steroid hormone attached to the drug binds its corresponding receptor, the formation of the receptor-ligand complex results in the internalization of the complex into the nucleus, thus resulting in nuclear translocation of the drug. Also disclosed is a conjugate (comprising the complex of the drug and the steroid hormone) bound to a polymer by spacers allowing for concurrent passive targeting to the tumor cell (afforded by attachment to the polymer by the EPR effect) and nuclear targeting of the conjugate (due to the presence of the steroid). Using a suitable degradable spacer allows for the release of free drug in the tumor and enhances nuclear targeting efficacy. The polymer can be further linked to a cellular targeting molecule, where the targeting molecule directs the polymer to specific cells. One may thus be able to effectively target drugs to the nucleus of tumor cells. With little or modifications, several therapeutic agents can be targeted using the invention.
Owner:UNIV OF UTAH RES FOUND
Cell death-inducing agent
InactiveUS20060275301A1Remarkable effectReduce molecular weightImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenCross-link
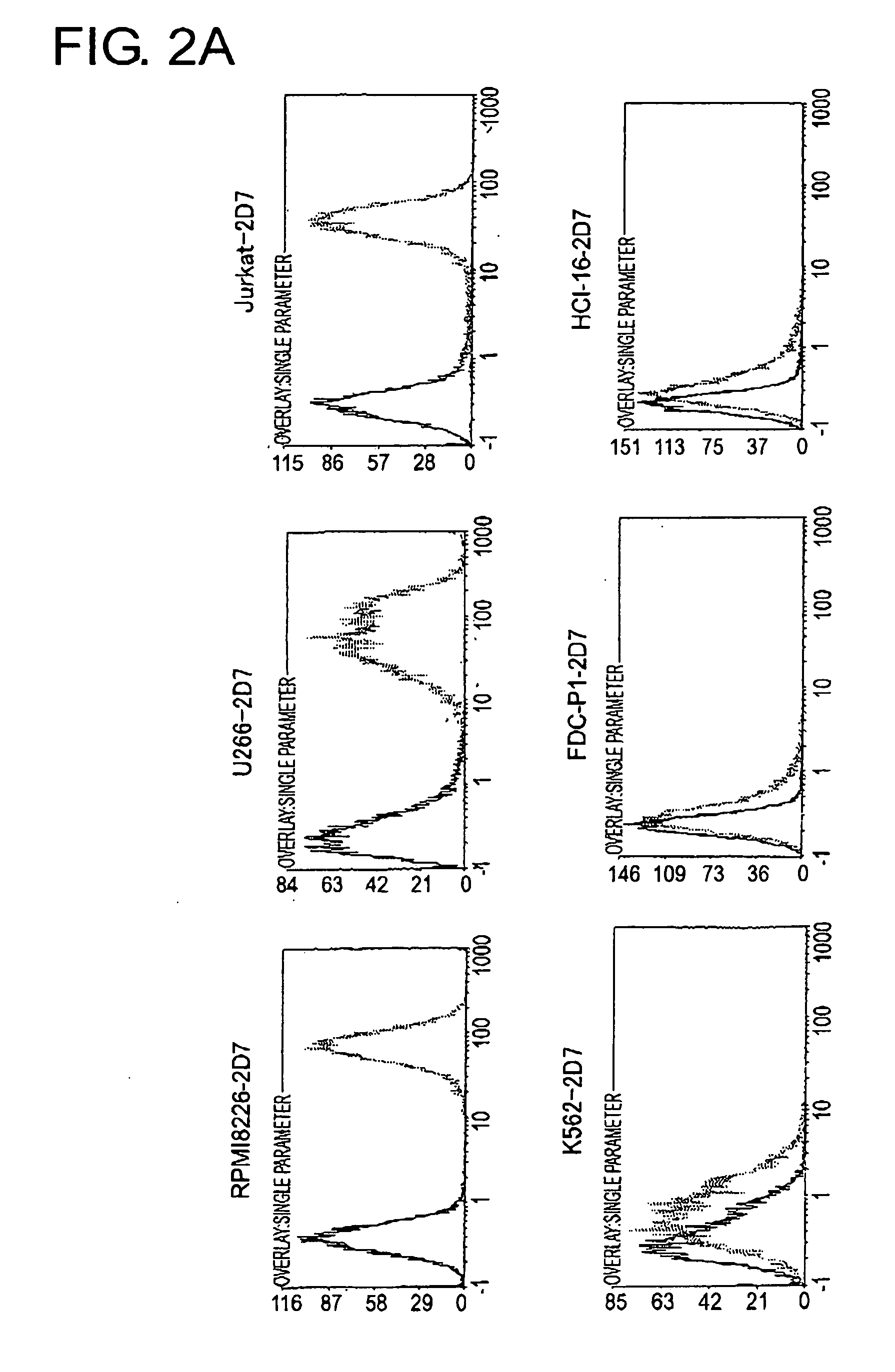
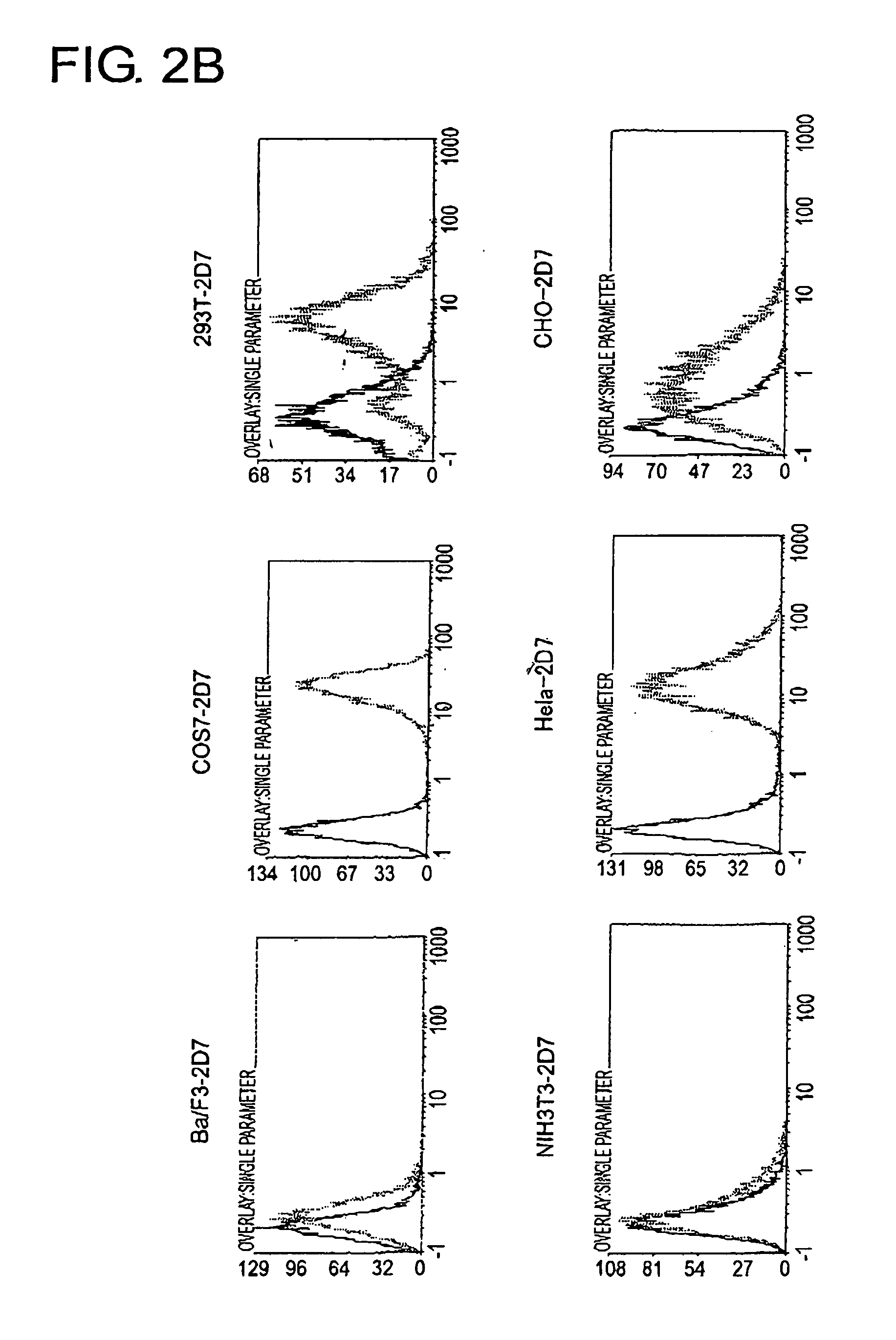
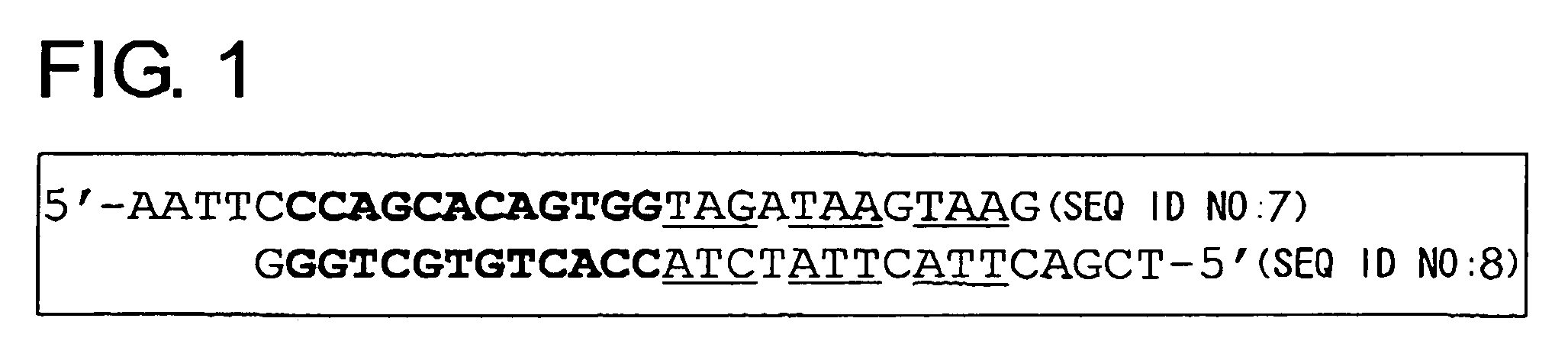
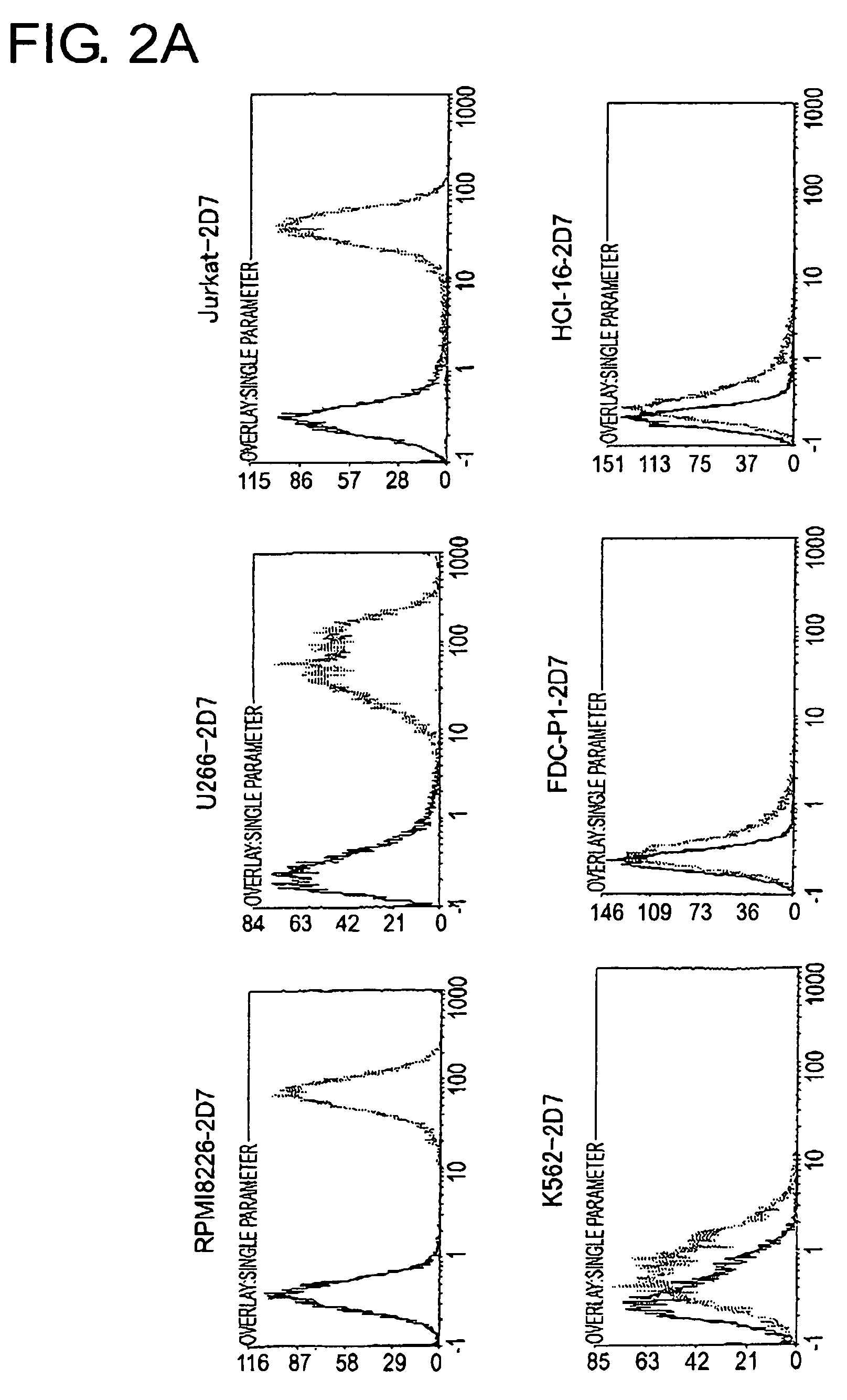
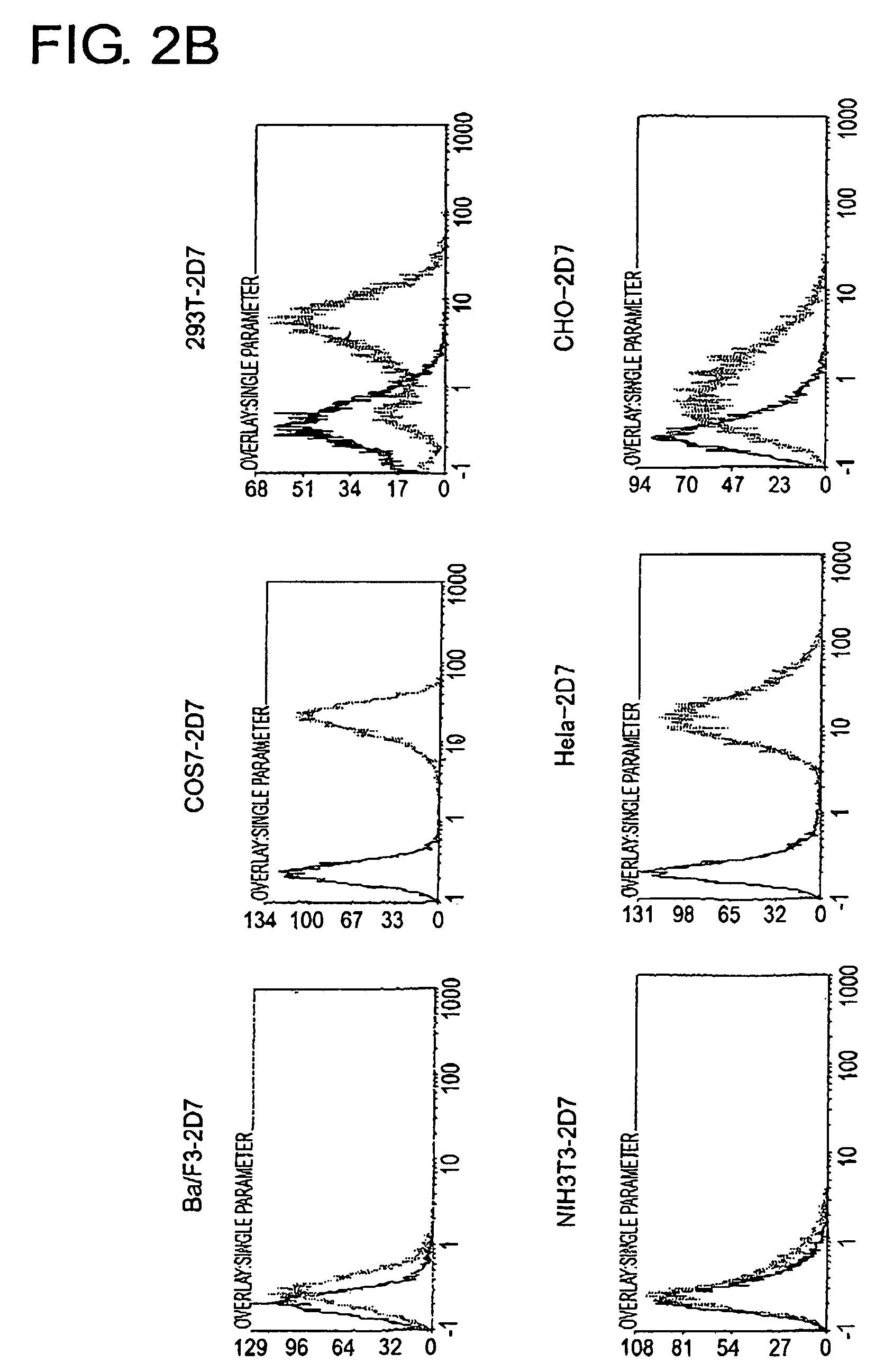
To identify antigens of the 2D7 antibody, the present inventors cloned the 2D7 antigen. The results suggested that the 2D7 antigen is an HLA class I molecule. Based on this finding, the present inventors examined whether the 2D7 antibody has cell death-inducing activity. Nuclei fragmentation was observed when the 2D7 antibody was cross-linked with another antibody, indicating that cell-death was induced. Further, diabodies of the 2D7 antibody were found to have very strong cell death-inducing activities, even without the addition of another antibody. These results indicate that minibodies of an HLA-recognizing antibody can be used as cell death-inducing agents.
Owner:CHUGAI PHARMA CO LTD
Deep lung pulmonary delivery of treprostinil
InactiveUS20120177693A1Slow onsetHigh selectivityBiocideDispersion deliveryTreprostinilIntensive care medicine
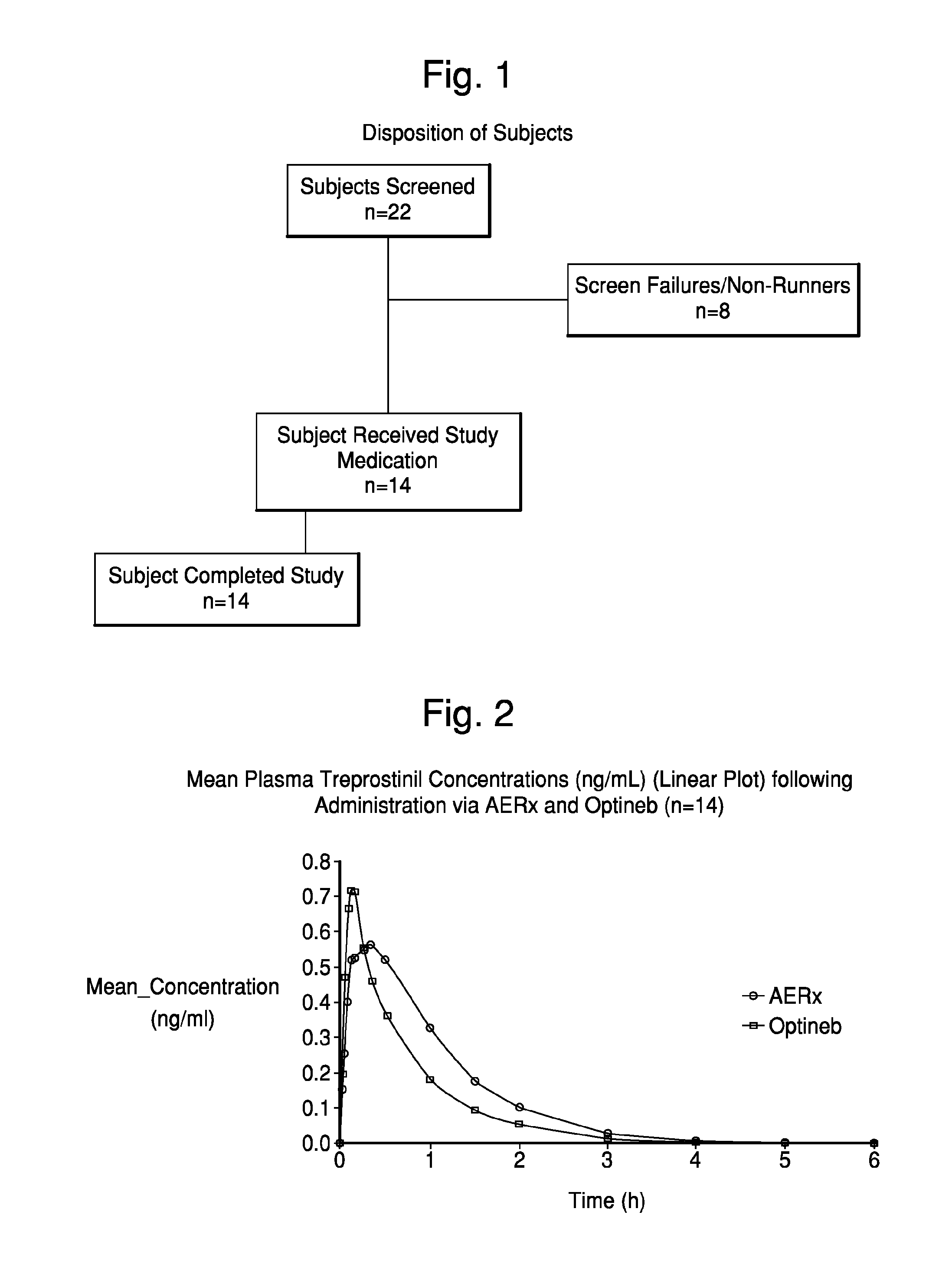
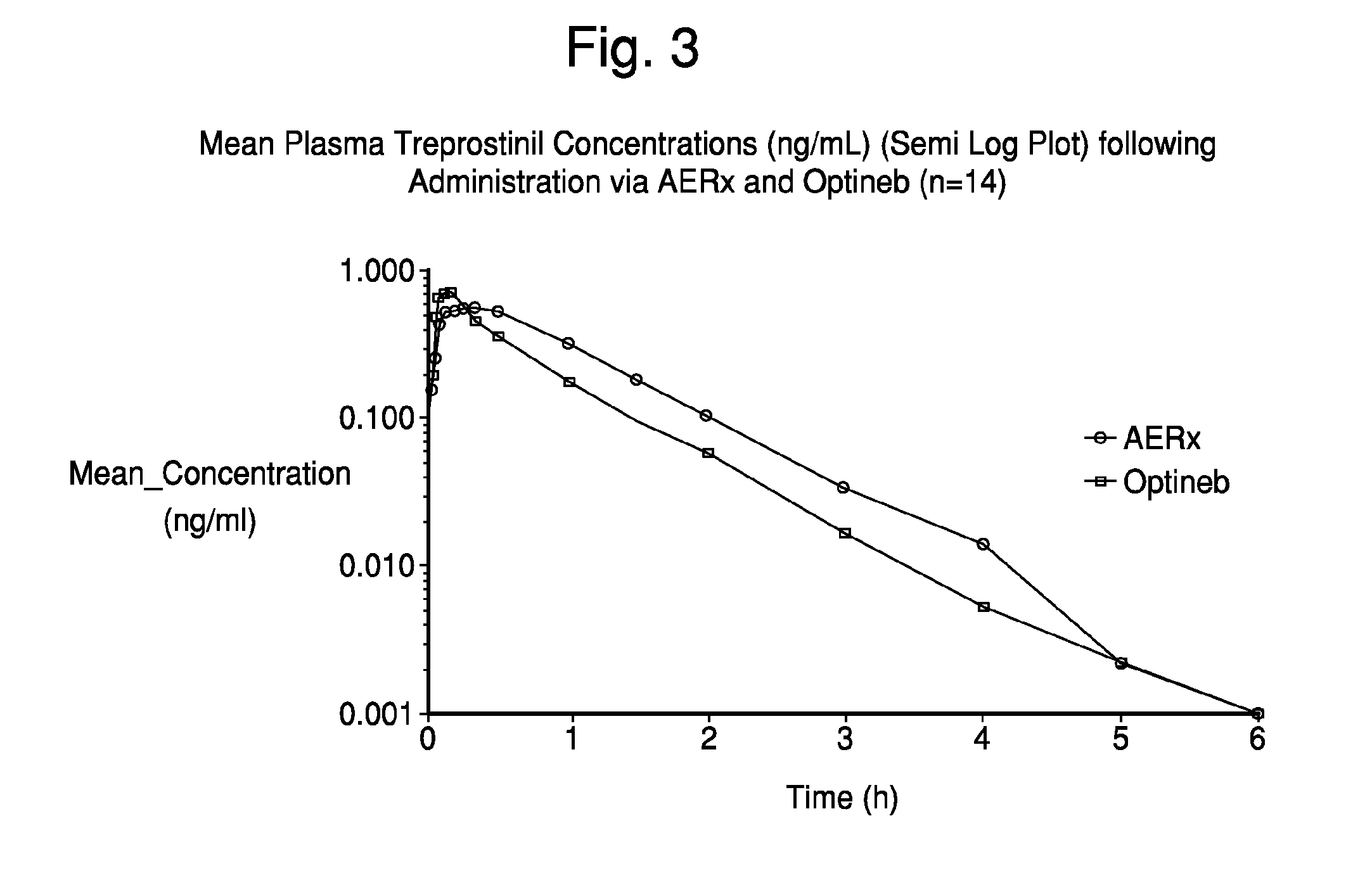
Administration of aerosolized Treprostinil formulations may provide a more homogeneous lung deposition of treprostinil, whereby making deep lung delivery possible.
Owner:ARADIGM
Chimeric factor vii molecules
ActiveUS20100330059A1Reduce riskLow affinityPeptide/protein ingredientsSemiconductor/solid-state device detailsFactor VIIImmunology
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Salts of benzimidazole compound and use thereof
InactiveUS20030181487A1Improve stabilityExcellent antiulcer actionAntibacterial agentsBiocideBarium saltMagnesium salt
Owner:TAKEDA PHARMA CO LTD
Composition comprising cocoa
InactiveUS20040005347A1Improve obesityReducing appetite and carbohydrate cravingBiocideOrganic active ingredientsDecreased LibidoAmine receptor
The invention pertains to a composition and a method for the treatment of mood disorders, in particular of treating, preventing or alleviate depression, mood disorders or insufficient mood, obesity, overweight, premenstrual syndrome, craving, carbohydrate craving, chocolate craving, menopausal complaints, erectile dysfunction and / or reduced libido. The composition contains cocoa or one or more of its pharmacologically active components, and a dopamine D2 receptor agonist.
Owner:NV NUTRICIA
Methods and compositions for treating a cell-proliferative disorder using CRE decoy oligomers, BCL-2 antisense oligomers, and hybrid oligomers thereof
InactiveUS20030176376A1Hinder and prevent bindingModulate transcriptional activitySugar derivativesPeptide/protein ingredientsDiseaseAbnormal tissue growth
The present invention is directed to hybrid oligomers comprising a cyclic AMP response element (CRE) sequence and a sequence that hybridizes to a bcl-2 pre-mRNA or mRNA, and pharmaceutical compositions comprising such hybrids. The present invention is also directed to the use of CRE decoy oligomers, comprising a CRE consensus sequence, and bcl-2 antisense oligomers in combination therapies, and the use of bcl-2 / CRE hybrid oligomers, to treat or prevent cell-proliferative related disorders, including hyperplasias, cancers, tumors and carcinomas. In one embodiment, the invention relates to therapeutic protocols comprising the administration of a CRE decoy oligomer and a bcl-2 antisense oligomer for the treatment of cell-proliferative related disorders. In another embodiment, the invention relates to therapeutic protocols comprising the administration of a bcl-2 antisense / CRE decoy (bcl-2 / CRE) hybrid oligomer for the treatment of cell-proliferative related disorders.
Owner:GENTA INC
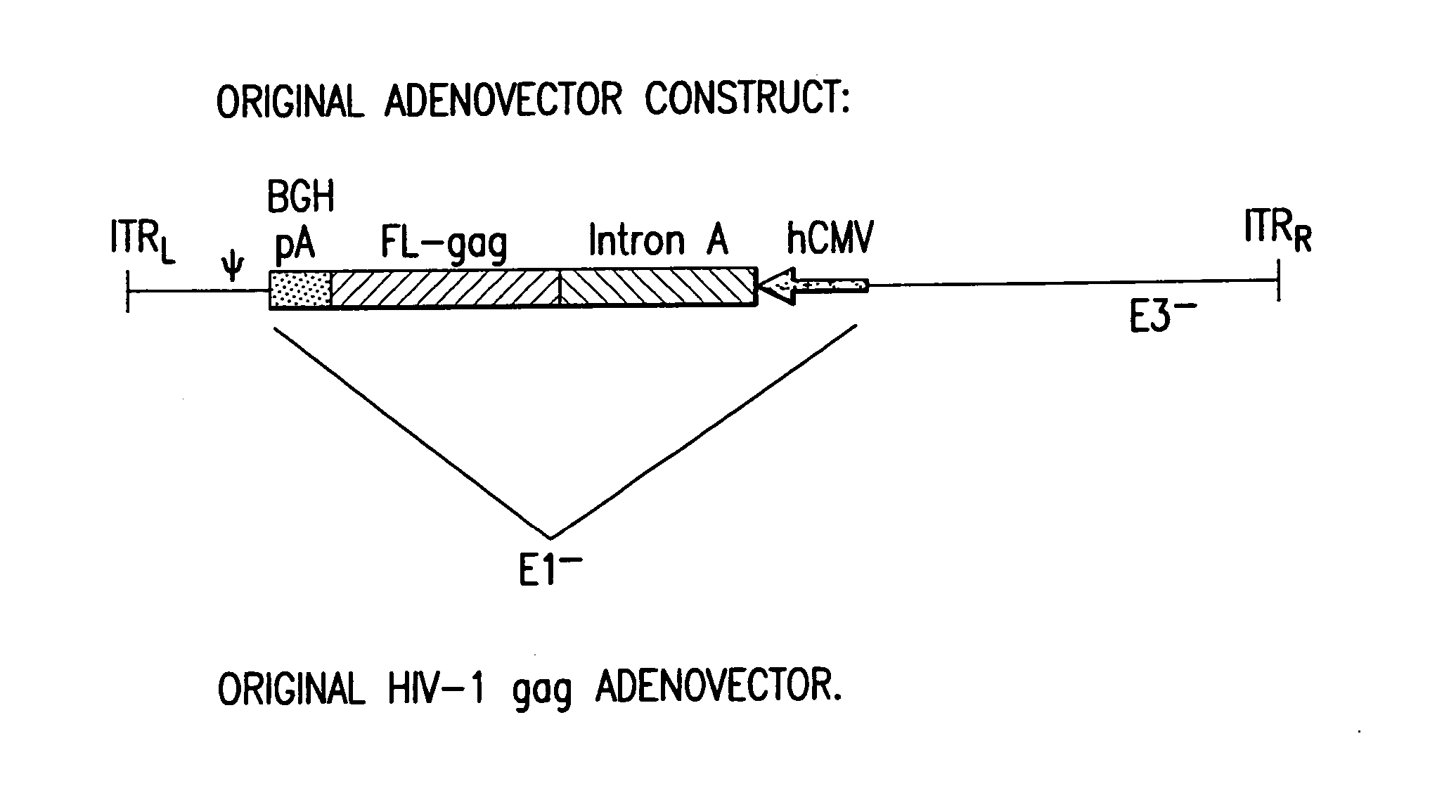
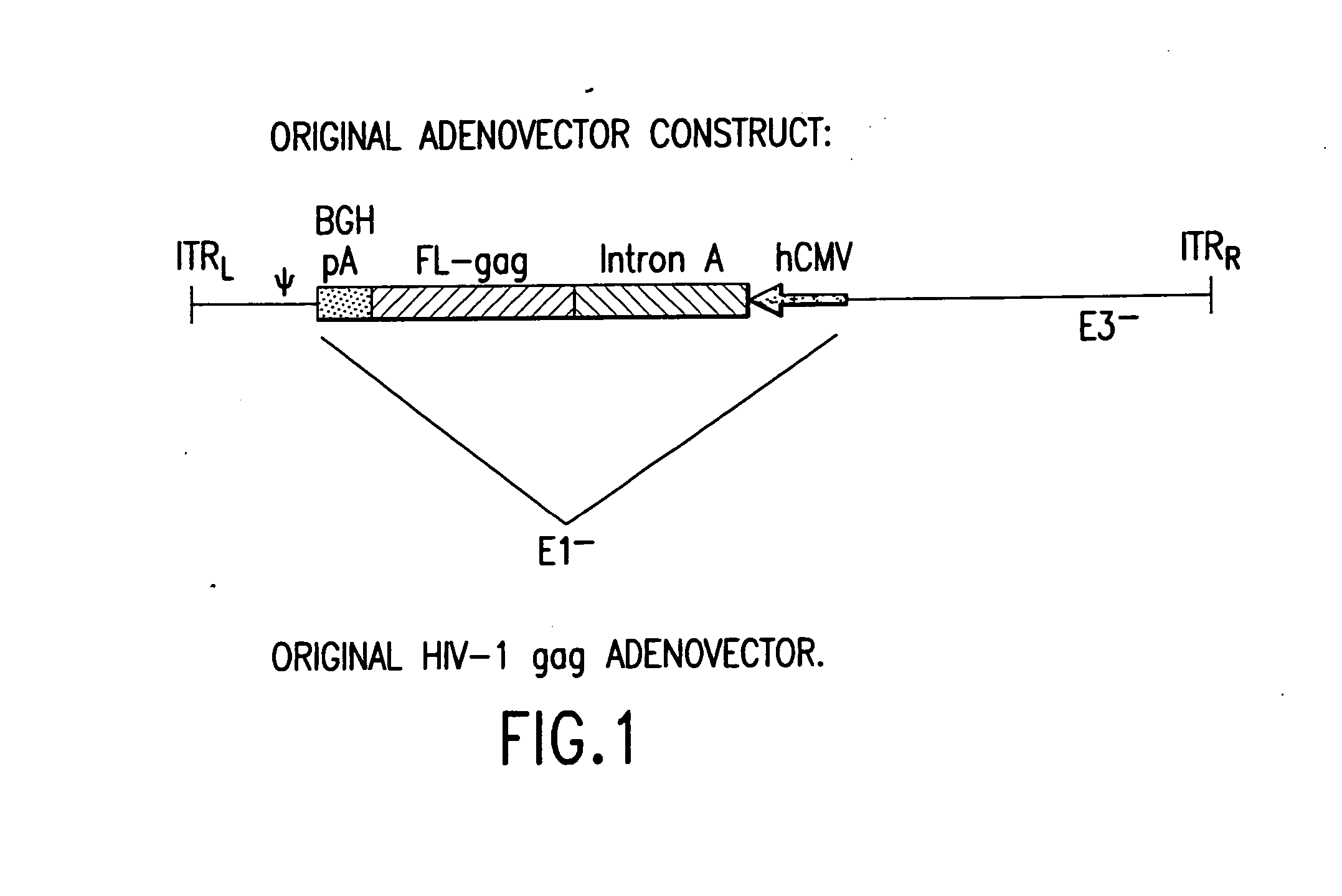
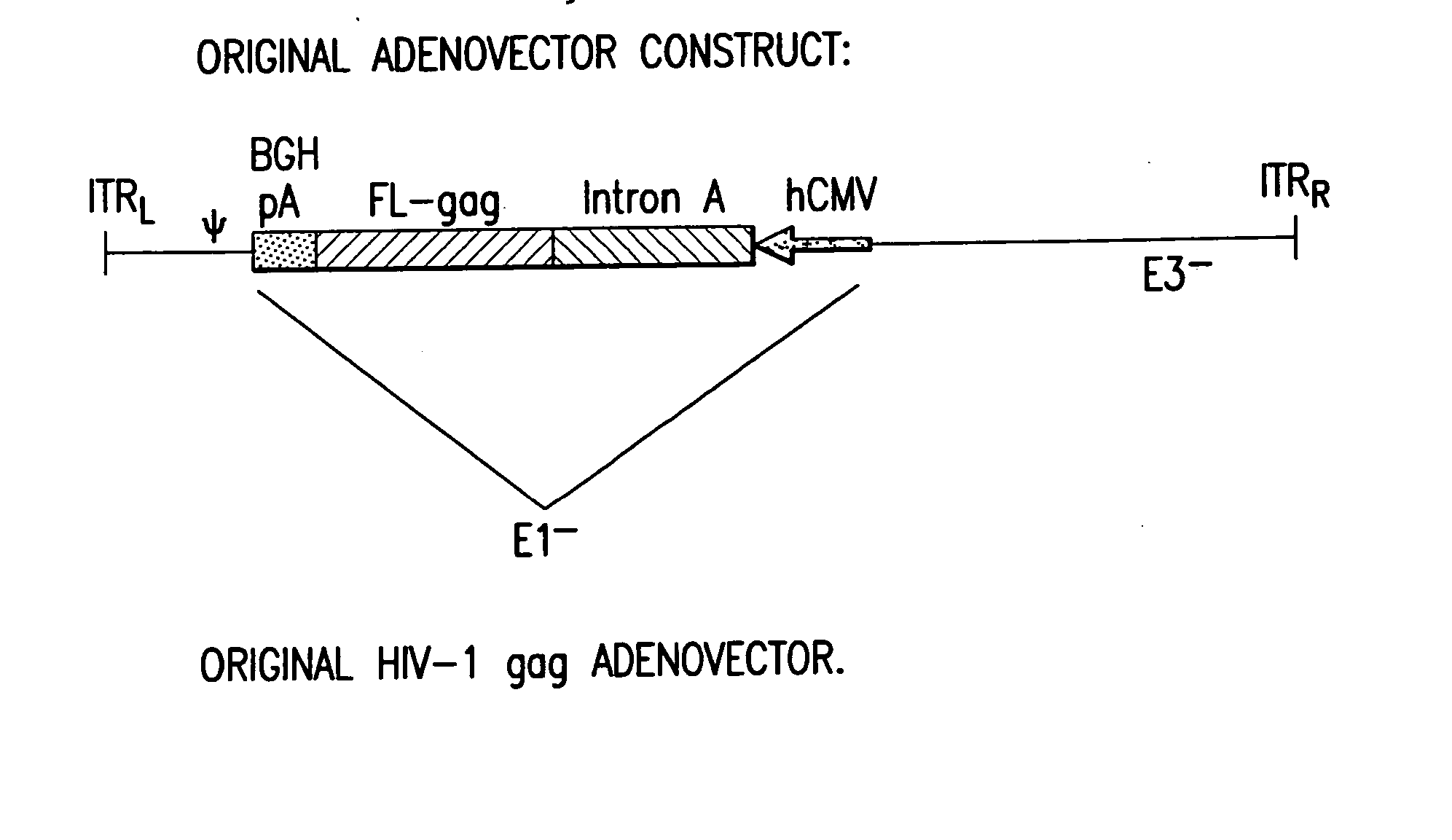
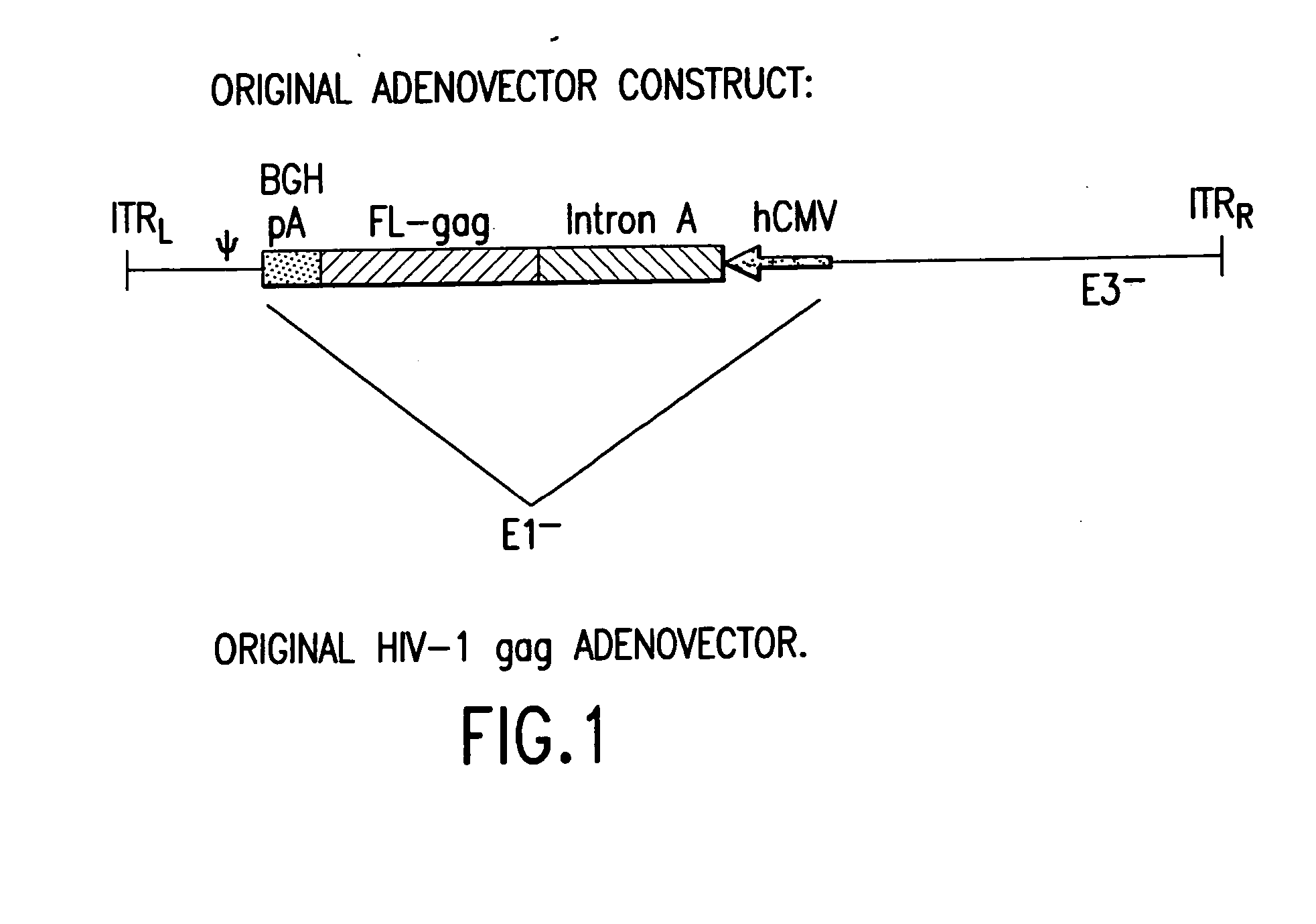
Enhanced first generation adenovirus vaccines expressing codon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070054395A1Genetically stableImprove featuresVectorsViral antigen ingredientsMammalReverse transcriptase
First generation adenoviral vectors and associated recombinant adenovirus-based HIV vaccines which show enhanced stability and growth properties and greater cellular-mediated immunity are described within this specification. These adenoviral vectors are utilized to generate and produce through cell culture various adenoviral-based HIV-1 vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof. These adenovirus vaccines, when directly introduced into living vertebrate tissue, preferably a mammalian host such as a human or a non-human mammal of commercial or domestic veterinary importance, express the HIV1-Gag, Pol and / or Nef protein or biologically modification thereof, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, encoding codon optimized HIV-1 Pol, derivatives of optimized HIV-1 Pol (including constructs wherein protease, reverse transcriptase, RNAse H and integrase activity of HIV-1 Pol is inactivated), HIV-1 Nef and derivatives of optimized HIV-1 Nef, including nef mutants which effect wild type characteristics of Nef, such as myristylation and down regulation of host CD4. The adenoviral vaccines of the present invention, when administered alone or in a combined modality regime, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
Methods and compositions for treating a cell-proliferative disorder using CRE decoy oligomers, BCL-2 antisense oligomers, and hybrid oligomers thereof
InactiveUS7060690B2Hinder and prevent bindingModulate transcriptional activitySugar derivativesPeptide/protein ingredientsDiseaseAbnormal tissue growth
The present invention is directed to hybrid oligomers comprising a cyclic AMP response element (CRE) sequence and a sequence that hybridizes to a bcl-2 pre-mRNA or mRNA, and pharmaceutical compositions comprising such hybrids. The present invention is also directed to the use of CRE decoy oligomers, comprising a CRE consensus sequence, and bcl-2 antisense oligomers in combination therapies, and the use of bcl-2 / CRE hybrid oligomers, to treat or prevent cell-proliferative related disorders, including hyperplasias, cancers, tumors and carcinomas. In one embodiment, the invention relates to therapeutic protocols comprising the administration of a CRE decoy oligomer and a bcl-2 antisense oligomer for the treatment of cell-proliferative related disorders. In another embodiment, the invention relates to therapeutic protocols comprising the administration of a bcl-2 antisense / CRE decoy (bcl-2 / CRE) hybrid oligomer for the treatment of cell-proliferative related disorders.
Owner:GENTA INC
Process for preducing mixed electrolyzed water
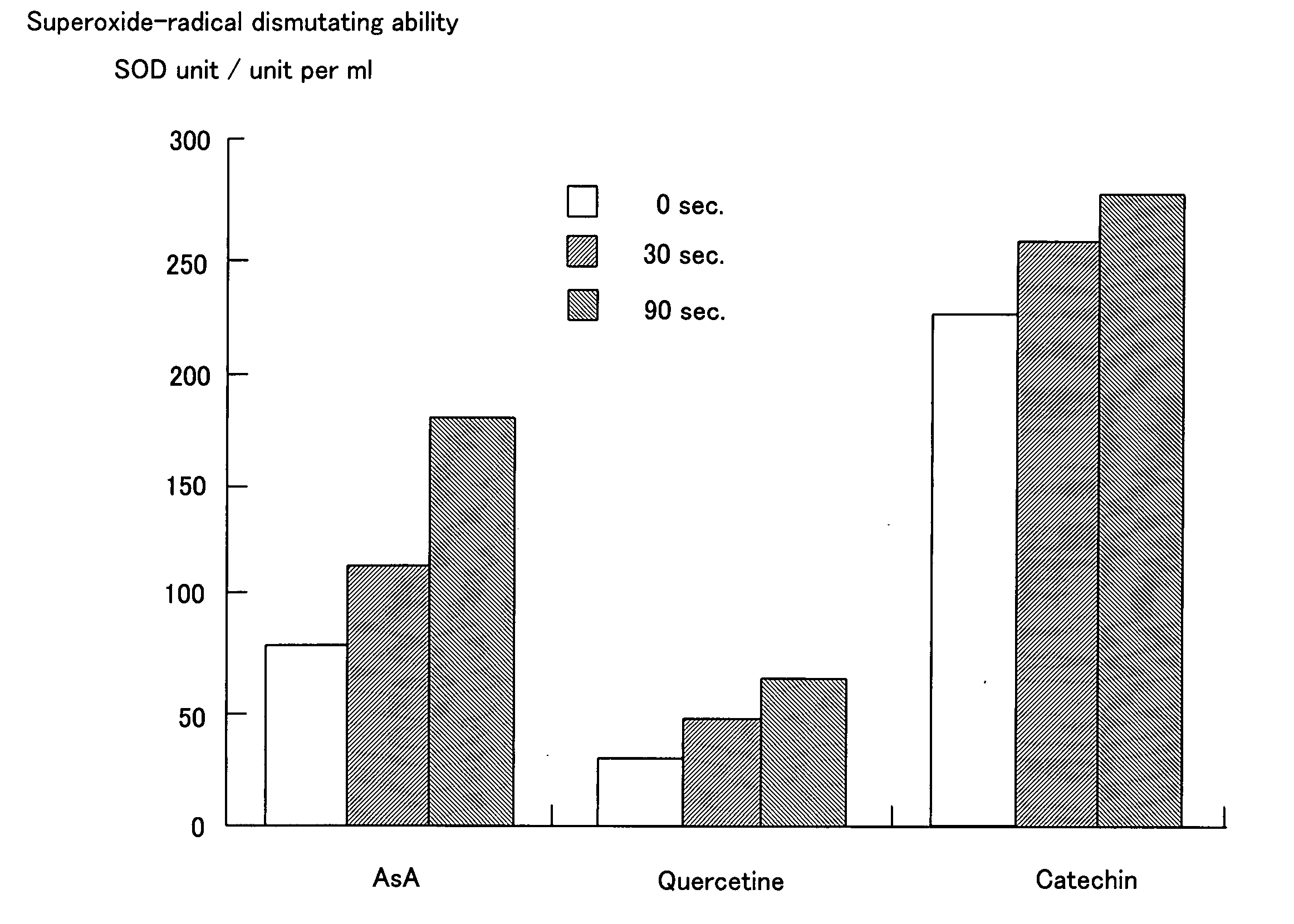
InactiveUS20060065544A1Efficient mixingImprove abilitiesWater treatment parameter controlPhotography auxillary processesInorganic saltsElectrolysis
This invention discloses a process for preparing a mixed electrolyzed water consisting of a cathodic and an anodic electrolyzed waters comprising the step of electrolyzing an aqueous solution of an organic electrolyte containing a water-soluble inorganic salt in less than 0.1 mM and an organic electrolyte in 1 to 50 mM which is fed into a non-diaphragm electrolytic bath comprising at least a pair of inactive electrodes separated from each other by an inter-electrode distance of 2 mm or less, wherein the aqueous solution of an organic electrolyte with pH equal to that of the mixed electrolyzed water prepared by electrolysis is neutralized with a titration volume less than that for the raw aqueous solution in neutralization titration with an aqueous solution of sodium hydroxide or has a higher dismutation activity to superoxide radical per mole than the raw aqueous solution.
Owner:MIKUNI CORP +1
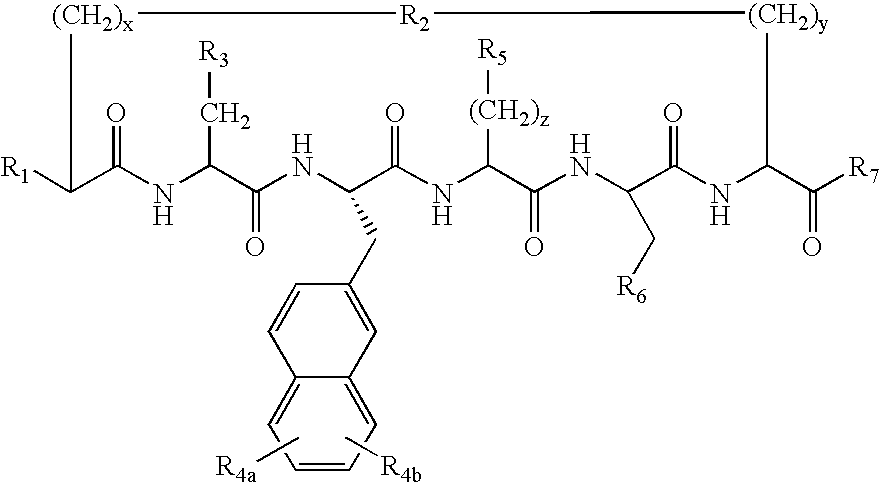
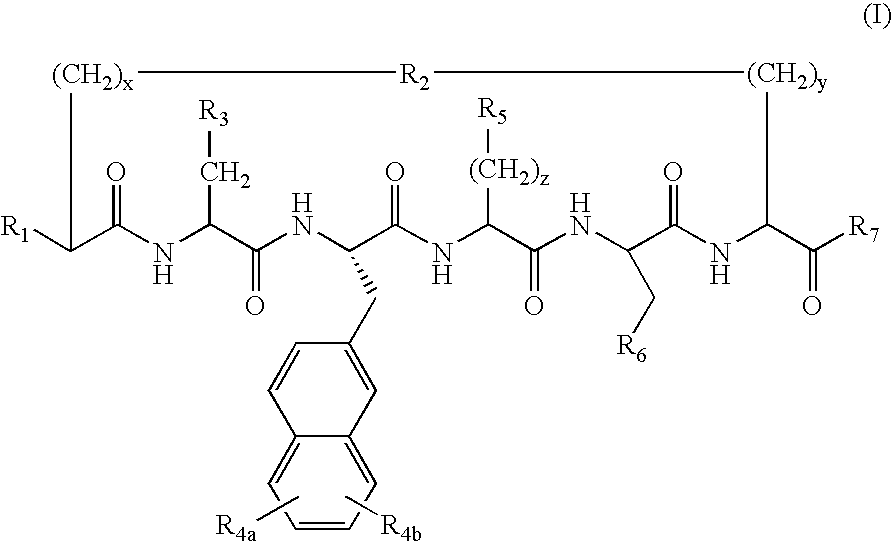
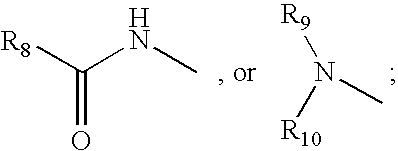
Cyclic peptides for treatment of cachexia
A highly selective melanocortin-4 receptor antagonist cyclic peptide of the formula where R1, R2, R3, R4a, R4b, R5, R6, R7, x, y and z are as defined in the specification, and a method of treating body weight disorders, including cachexia, sarcopenia and wasting syndrome or disease, and treating inflammation and immune disorders.
Owner:PALATIN TECH INC
Composition comprising cocoa
InactiveUS20020172732A1Reduce appetiteReducing carbohydrate cravingBiocideOrganic active ingredientsPhysiologyAdditive ingredient
The invention pertains to a composition and a method for the treatment of mood disorders, in particular of treating, preventing or alleviating depression, mood disorders or insufficient mood, obesity, overweight, premenstrual syndrome, craving, carbohydrate craving, chocolate craving, menopausal complaints, erectile dysfunction and / or reduced libido, The composition contains cocoa or one or more of its pharmacologically active components, and a dopamine D2 receptor agonist.
Owner:NUTRICIA
Enhanced first generation adenovirus vaccines expressing condon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070077257A1Improved cellular-mediated immune responseLow transmission rateViral antigen ingredientsVirus peptidesTreatment effectNucleotide
First generation adenoviral vectors and-recombinant adenovirus-based HIV vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof are described. The adenovirus vaccines, when directly introduced into living vertebrate tissue, express the relevant proteins, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, HIV-1 Pol, HIV-1 Nef, and derivatives thereof. The adenoviral vaccines of the present invention, alone or in combination, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
Novel msln targeting DNA vaccine for cancer immunotherapy
InactiveUS20160317634A1Optimize treatment planStrong immune responseBacteriaCancer antigen ingredientsRecombinant DNADNA
The present invention relates to an attenuated mutant strain of Salmonella comprising a recombinant DNA molecule encoding Mesothelin. In particular, the present invention relates to the use of said attenuated mutant strain of Salmonella in cancer immunotherapy.
Owner:VAXIMM GMBH
New drug
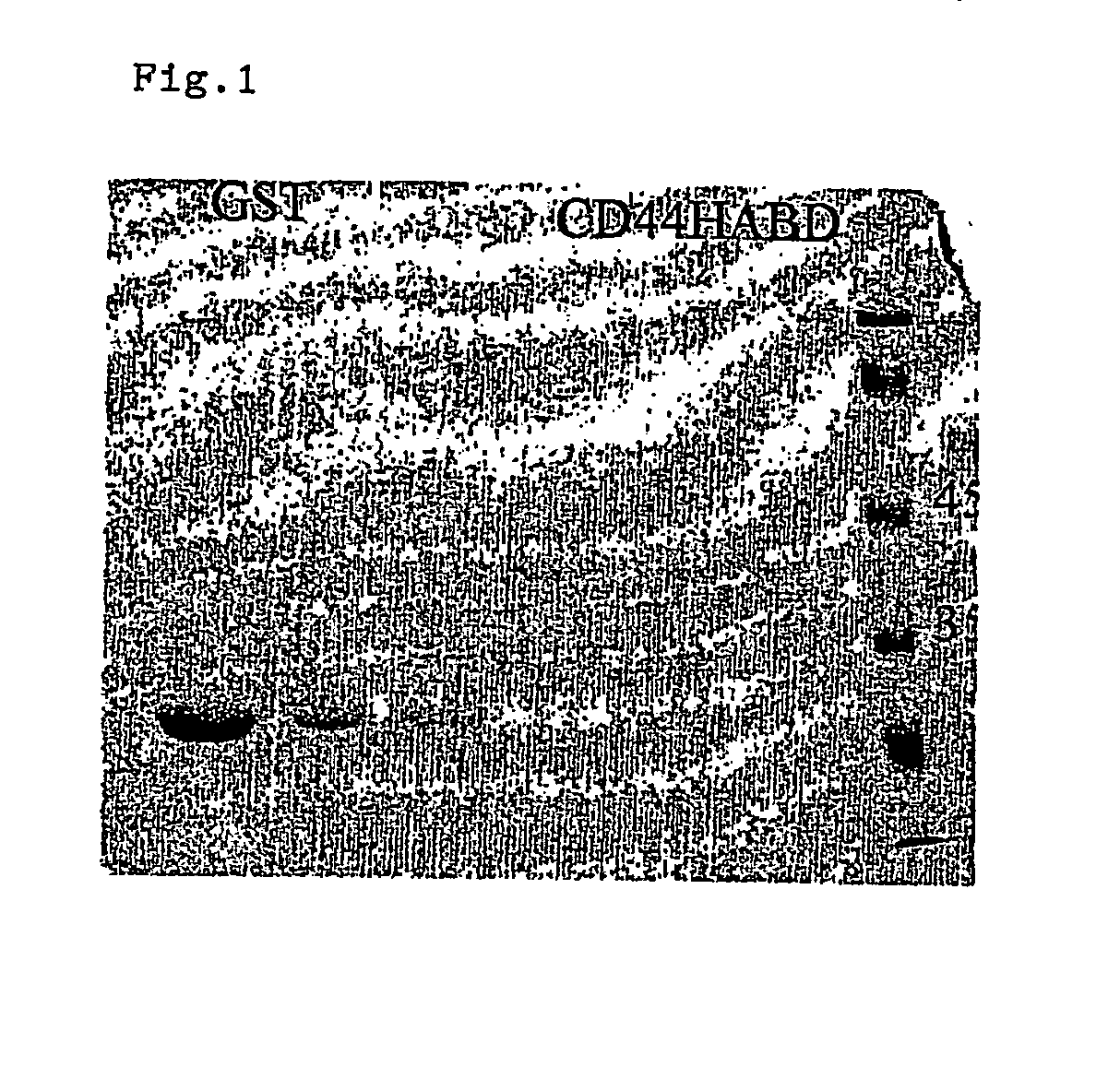
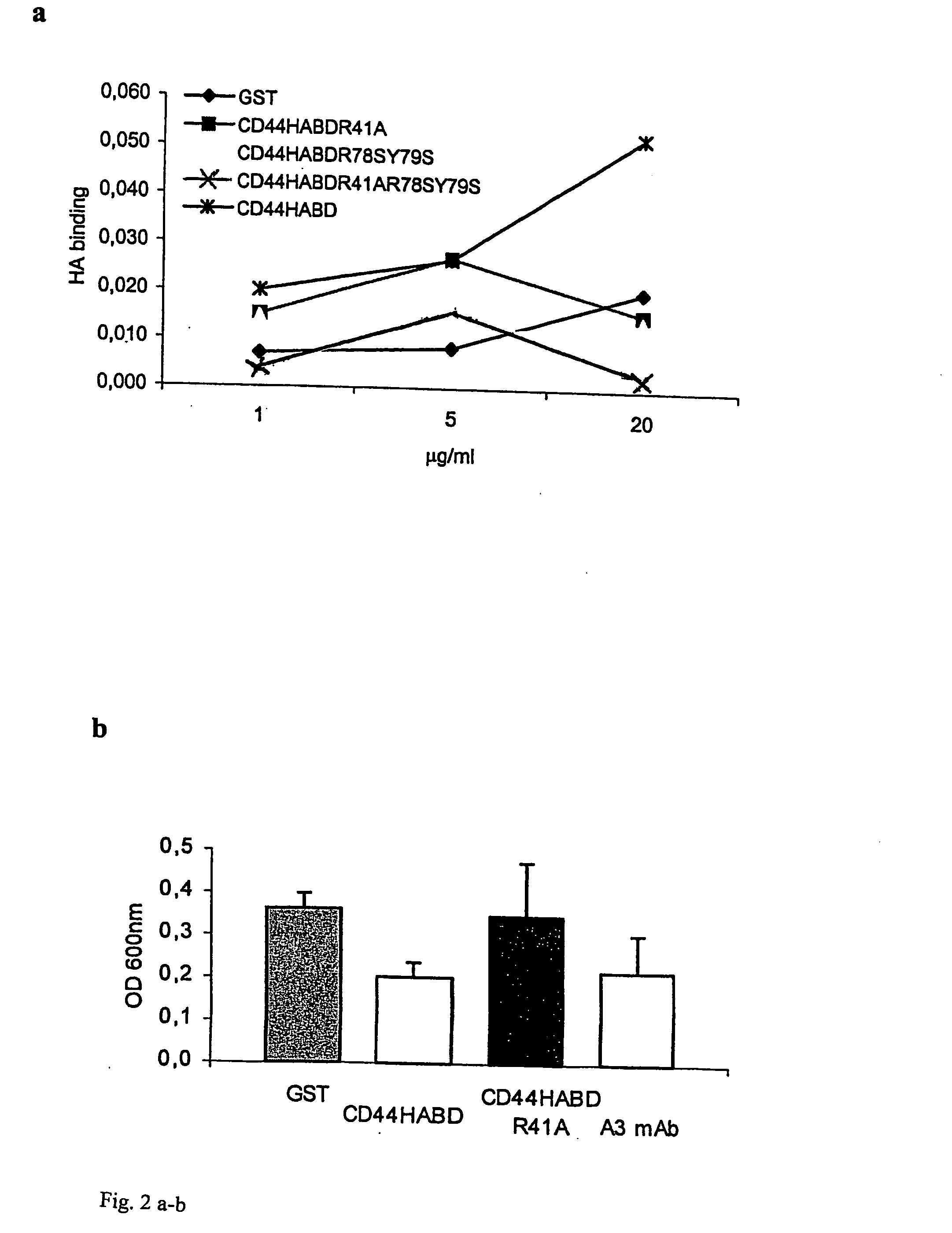
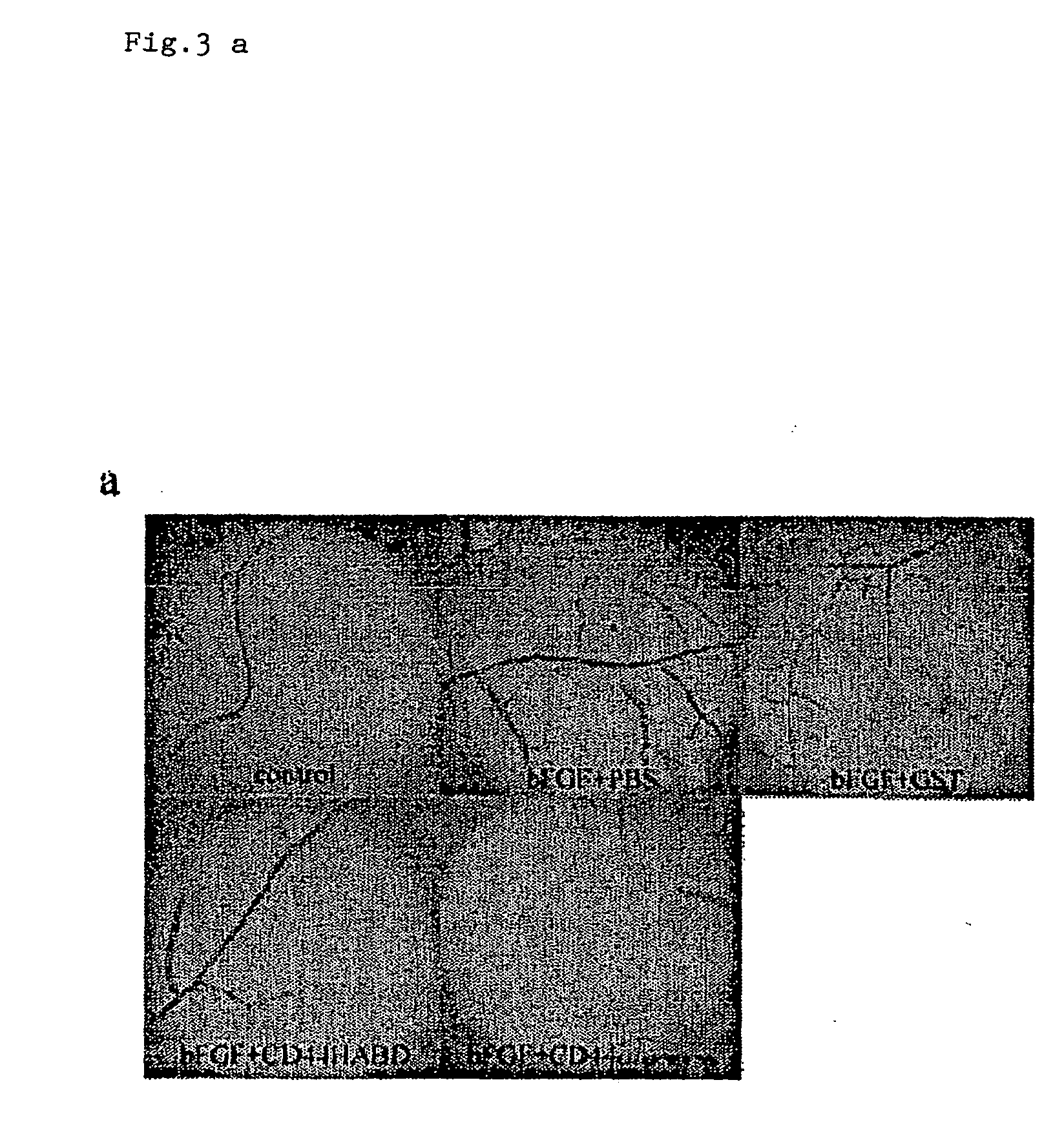
InactiveUS20050054593A1Growth inhibitionKeep for a long timePeptide/protein ingredientsAntibody mimetics/scaffoldsLymphatic SpreadMurine fibrosarcoma
CD44, the receptor for hyaluronic acid, has complex functions in cellular physiology, cell migration and tumour metastasis. The inventors have previously found that human CD44 receptor overexpression in mouse fibrosarcoma cells inhibits subcutaneous tumour growth in mice [Kogerman et al., Oncogene 1997; 15:1407-16; Kogerman et al., Clin Exp Metastasis 1998; 16:83-93]. Here it is demonstrated that a tumour growth inhibitory effect of CD44 is caused by block of angiogenesis. Furthermore, the inventors have found that soluble recombinant CD44 hyaluronic acid binding domain (CD44HABD) inhibits angiogenesis in vivo in cLick and mouse and thereby inhibits human tumour growth of various origins. The anti-angiogenic effect of CD44-HABD is independent of hyaluronic acid (HA) binding, since non-HA-binding mutants of CD44HABD still maintain anti-angiogenic properties. The invention discloses soluble CD44 recombinant proteins as a novel class of angiogenesis inhibitors based on targeting of vascular cell surface receptor. A method of block of angiogenesis and treatment of human tumours using recombinant CD44 proteins as well as their analogues is disclosed. As a further embodiment of the invention, methods for screening for new drug targets using CD44 recombinant proteins and their analogues is presented.
Owner:CELECURE
Cell death-inducing agent
InactiveUS8158385B2Reduce molecular weightShort timeImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenCell Death Induction
To identify antigens of the 2D7 antibody, the present inventors cloned the 2D7 antigen. The results suggested that the 2D7 antigen is an HLA class I molecule. Based on this finding, the present inventors examined whether the 2D7 antibody has cell death-inducing activity. Nuclei fragmentation was observed when the 2D7 antibody was cross-linked with another antibody, indicating that cell-death was induced. Further, diabodies of the 2D7 antibody were found to have very strong cell death-inducing activities, even without the addition of another antibody. These results indicate that minibodies of an HLA-recognizing antibody can be used as cell death-inducing agents.
Owner:CHUGAI PHARMA CO LTD
39-desmethoxy-39-methyl derivatives of rapamycin
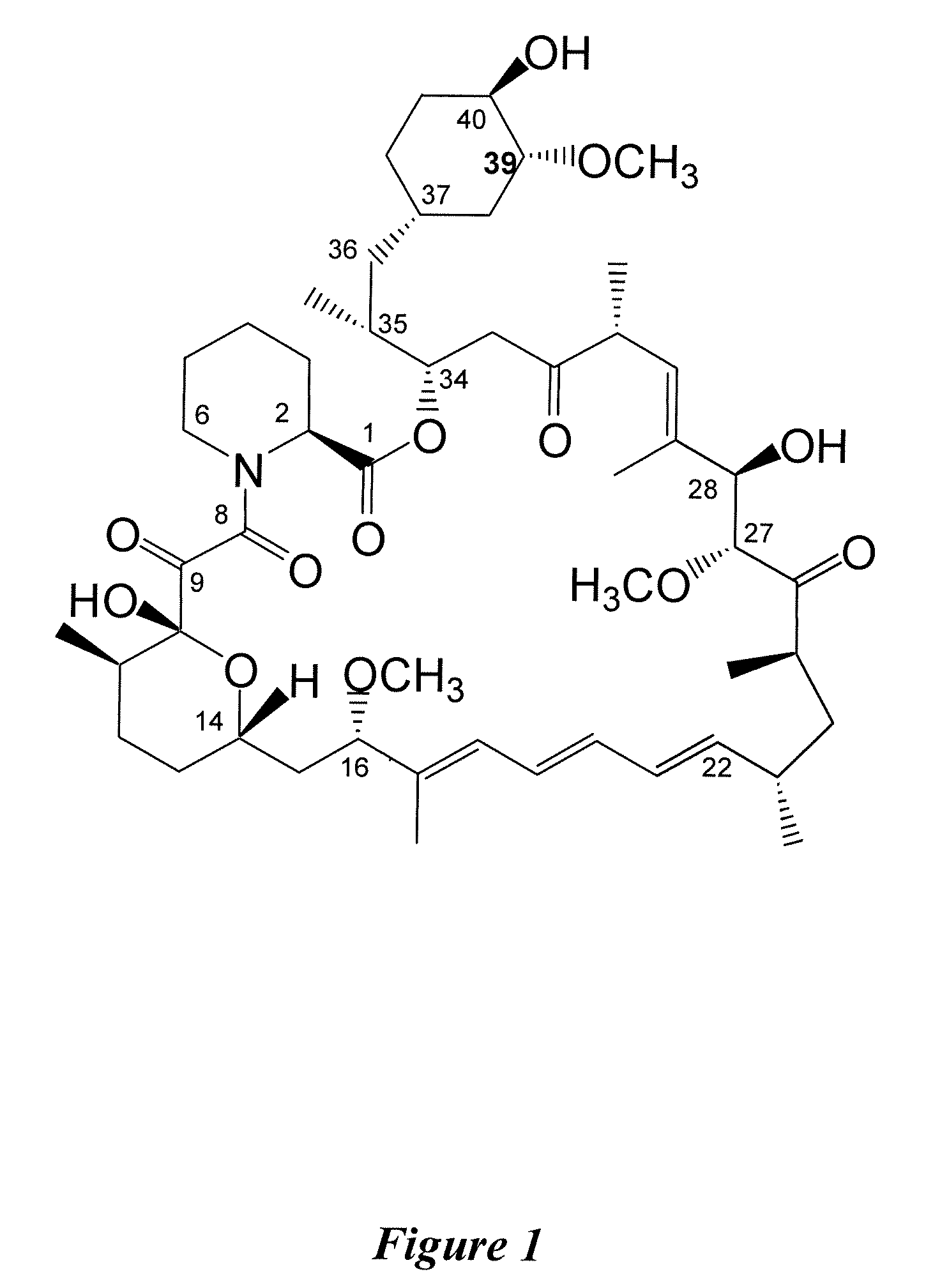
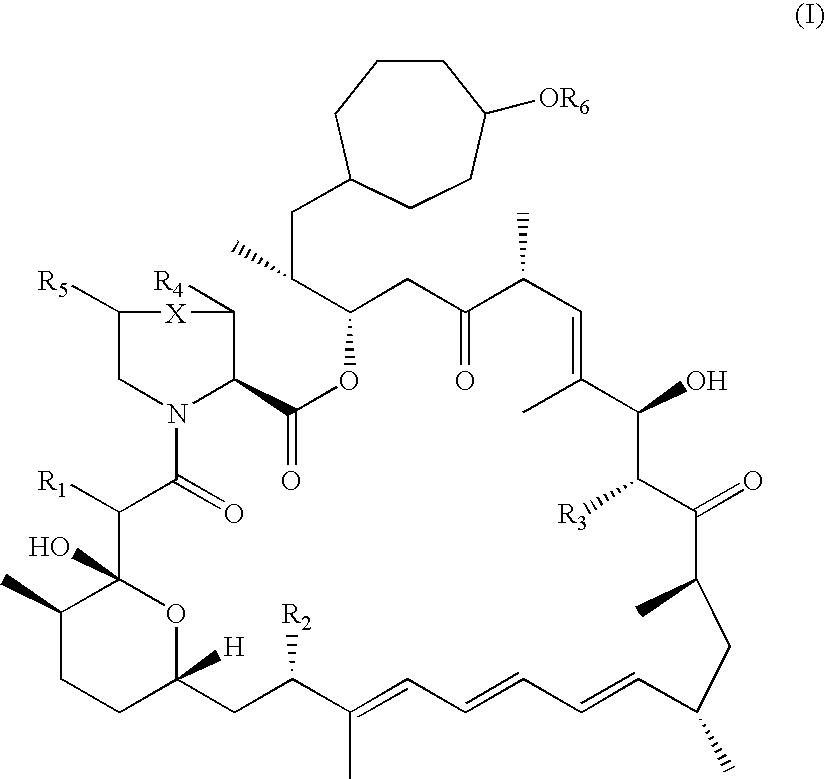
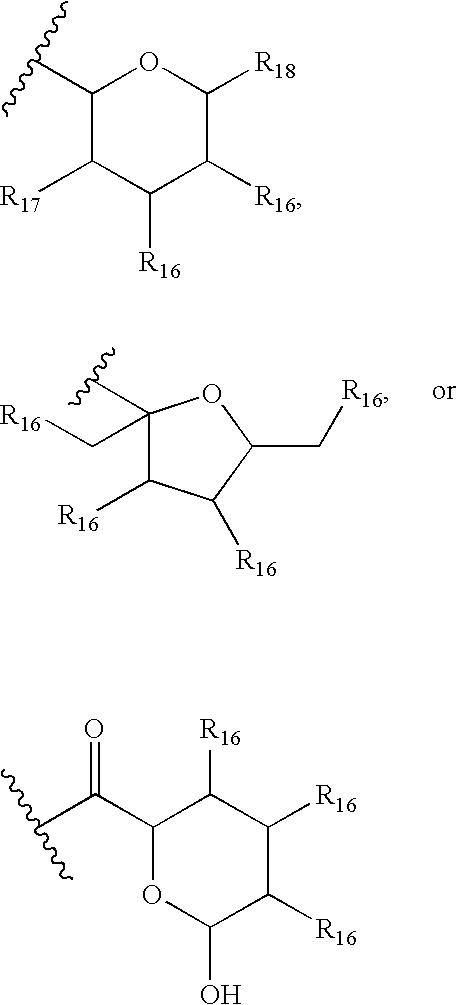
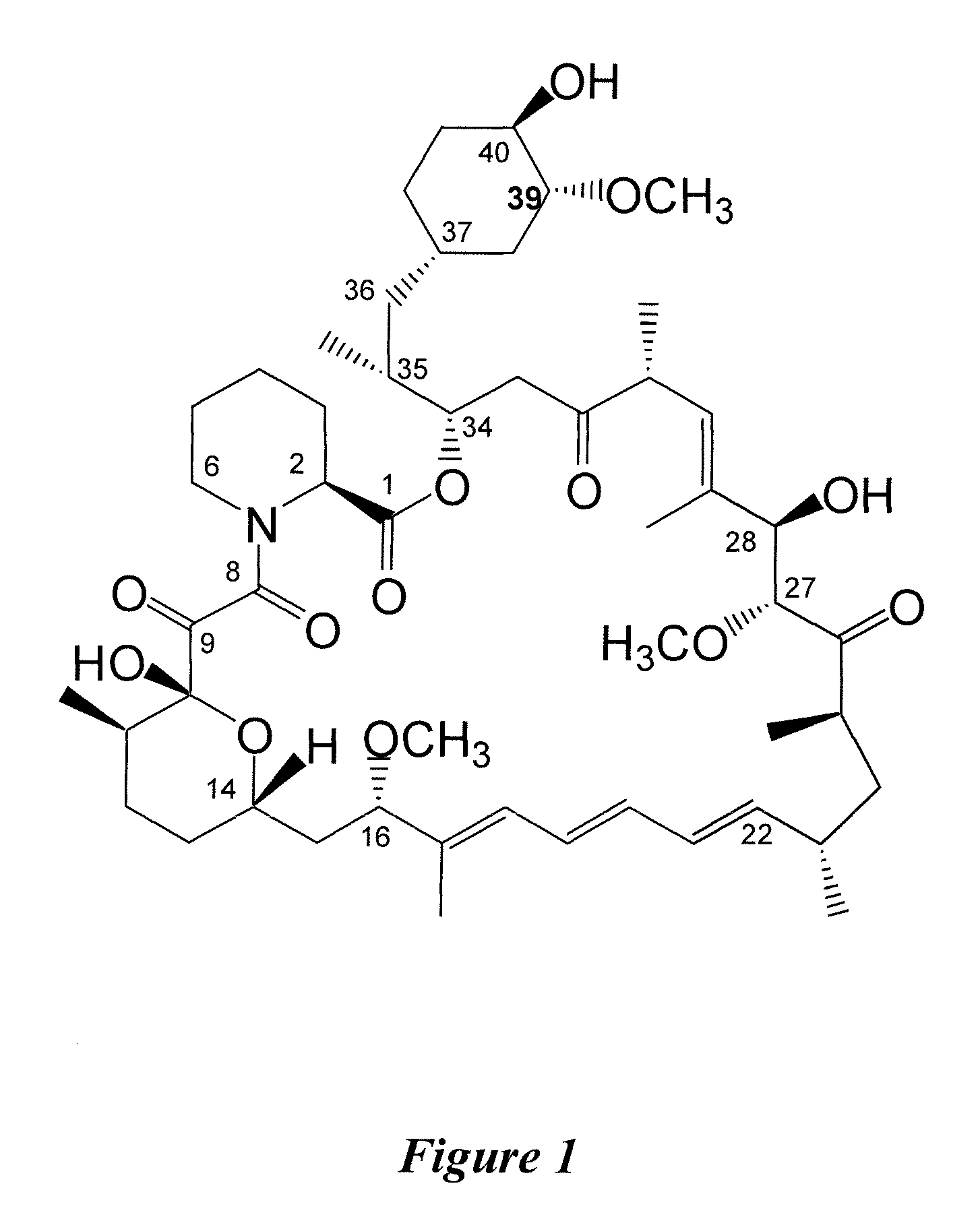
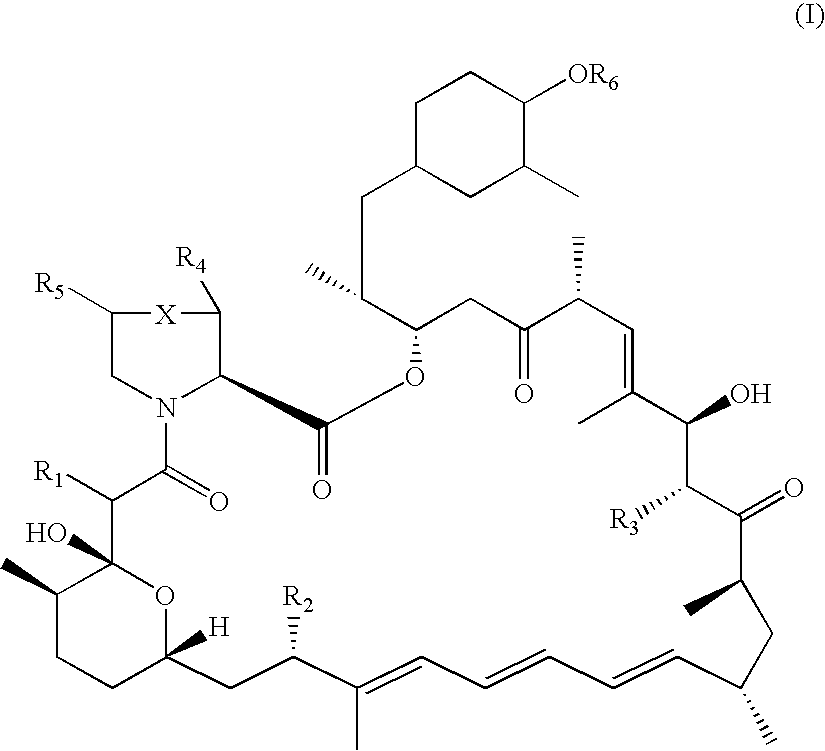
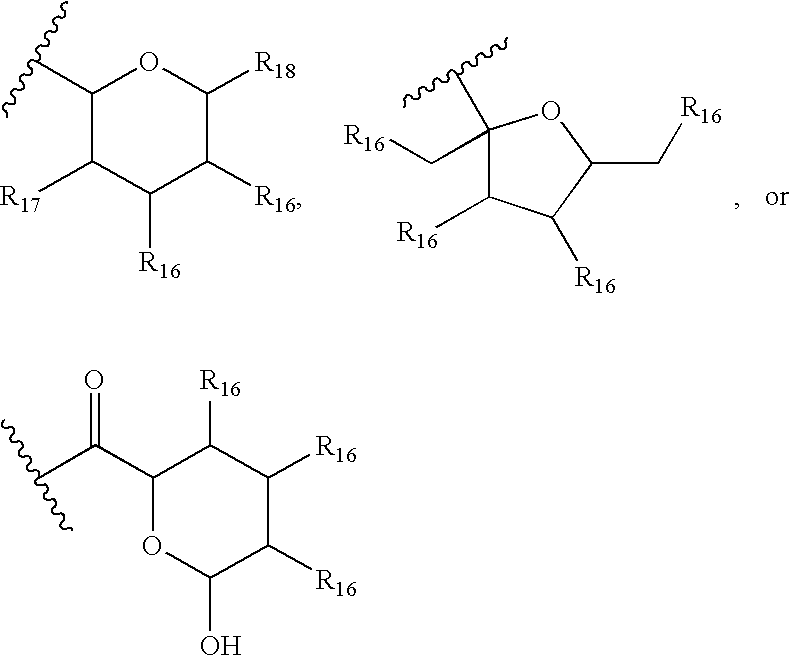
InactiveUS7897608B2Low in DOEliminate side effectsBiocideSenses disorderImmunologic disordersFibrosis
The present invention relates to novel 39-desmethoxy-39-methylrapamycin derivatives, methods for their production, and uses thereof. In a further aspect the present invention provides for the use of these 39-desmethoxy-39 -methylrapamycin derivatives in the treatment of cancer and / or B-cell malignancies, the induction or maintenance of immunosuppression, the treatment of transplantation rejection, graft vs. host disease, autoimmune disorders, diseases of inflammation, vascular disease and fibrotic diseases, the stimulation of neuronal regeneration or the treatment of fungal infections.
Owner:BIOTICA TECH
Inhibition of vacuolar proton ATPase activity and/or the modulation of acidic organelle function sensitizes cells to radiation, chemotherapy and biological agents
InactiveUS6982252B2Reduced dosDiminishes unwanted damageBiocideCompound screeningSensitized cellCancer cell
The present invention relates to methods and compositions for inhibiting cell survival and / or promoting cell death following exposure to cytotoxic agents and stress such as radiation or chemotherapy exposure through inhibition of V-H+-ATPase. In particular, the formation and / or acidification of acidic vesicular organelles (AVOs) may be prevented or decreased by inhibiting the activity of vacuolar proton ATPase (“V-H+-ATPase”). The methods and compositions of the invention are based on the observation that (i) following irradiation surviving cancer cells accumulate AVOs and that their acidification is mediated by V-H+-ATPase; (ii) surviving colonies of cells contain higher levels of AVOs; and (iii) inhibition of V-H+-ATPase decreases the clonogenic survival of cells irradiated or exposed to chemotherapeutic agents. These observations led to the conclusion that V-H+-ATPase activity and AVO function serve to protect cells from radiation and chemotherapy damage. In addition, agents such as bFGF, TNF-α, PMA, rapamycin and tamoxifen were shown to be inducers of acidic organelle formation. Therefore signal transduction pathways mediated by these agents provide targets for drug screening assays designed to identify inhibitors of V-H+-ATPase activity and AVO formation / acidification. The present invention may be used to treat cancer subjects through sensitization of neoplastic cells to the toxic effects of radiation and chemotherapy.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Salmonella-based vectors for cancer immunotherapy targeting wilms' tumor gene wt1
ActiveUS20160068801A1Optimize treatment planAttenuated <i>SalmonellaTumor rejection antigen precursorsBacteriaSalmonella SenftenbergImmune therapy
The present invention relates to an attenuated mutant strain of Salmonella comprising a recombinant DNA molecule encoding Wilms' tumor Protein 1. In particular, the present invention relates to the use of said attenuated mutant strain of Salmonella in cancer immunotherapy.
Owner:VAXIMM
Methods for improving therapeutic effectiveness of treatment of vascularization disorders
InactiveUS6020308AImprove efficiencyLow in DOBiocidePeptide/protein ingredientsVascular diseaseDisease
The present invention is directed to the use of an inhibitor of NO activity, such as a nitric oxide scavenger or an NO synthase inhibitor, as as an adjunct to treatment of inappropriate tissue vascularization disorders.
Owner:DUKE UNIV +2
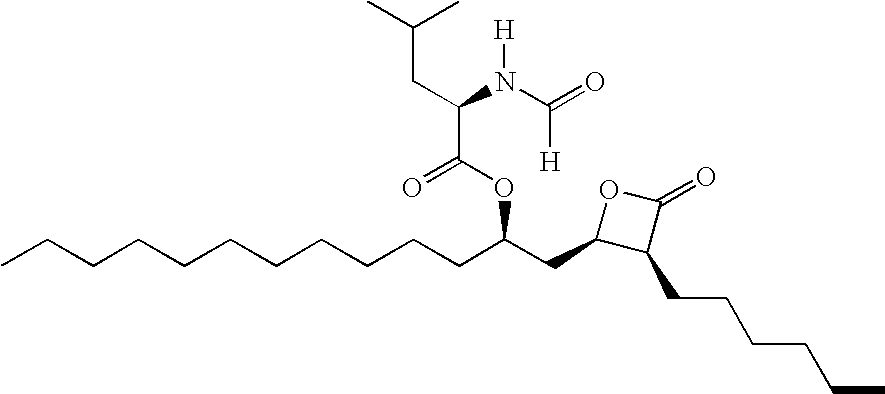
[[2-(Amino-3,4-dioxo-1-cyclobuten-1-yl)amino]alkyl]-acid derivatives for the treatment of pain
This invention provides a method of treating pain in a mammal that includes administering to a mammal in need of such treatment a pain treating effective amount of a compound of the formula (I): where R1 is H, alkyl or phenylalkyl; R2 is H, alkyl, alkenyl or phenylalkyl; or R1 and R2 taken together as Z are -CH2C-H2-, -CH2C(R6)(R7)CH2- or -CH2C(R8)(R9)-C(R10)(R11)CH2-, where R6, R8 and R10 are, independently, H, alkyl or hydroxyl and R7, R9 and R11 are, independently, H or alkyl; A is alkylene or alkenylene; X is CO2R3, P(O)(OR4)(OR5), 3,5-dioxo-1,2,4-oxadiazolidin-2-yl or 5-tetrazolyl in which R3, R4 and R5 are, independently, H or alkyl, or a pharmaceutically acceptable salt thereof. The present invention also provides pharmaceutical compositions for treating pain containing a pain treating effective amount of the compound of formula (I).
Owner:WYETH LLC
Methods and devices for measuring the concentration of an additive
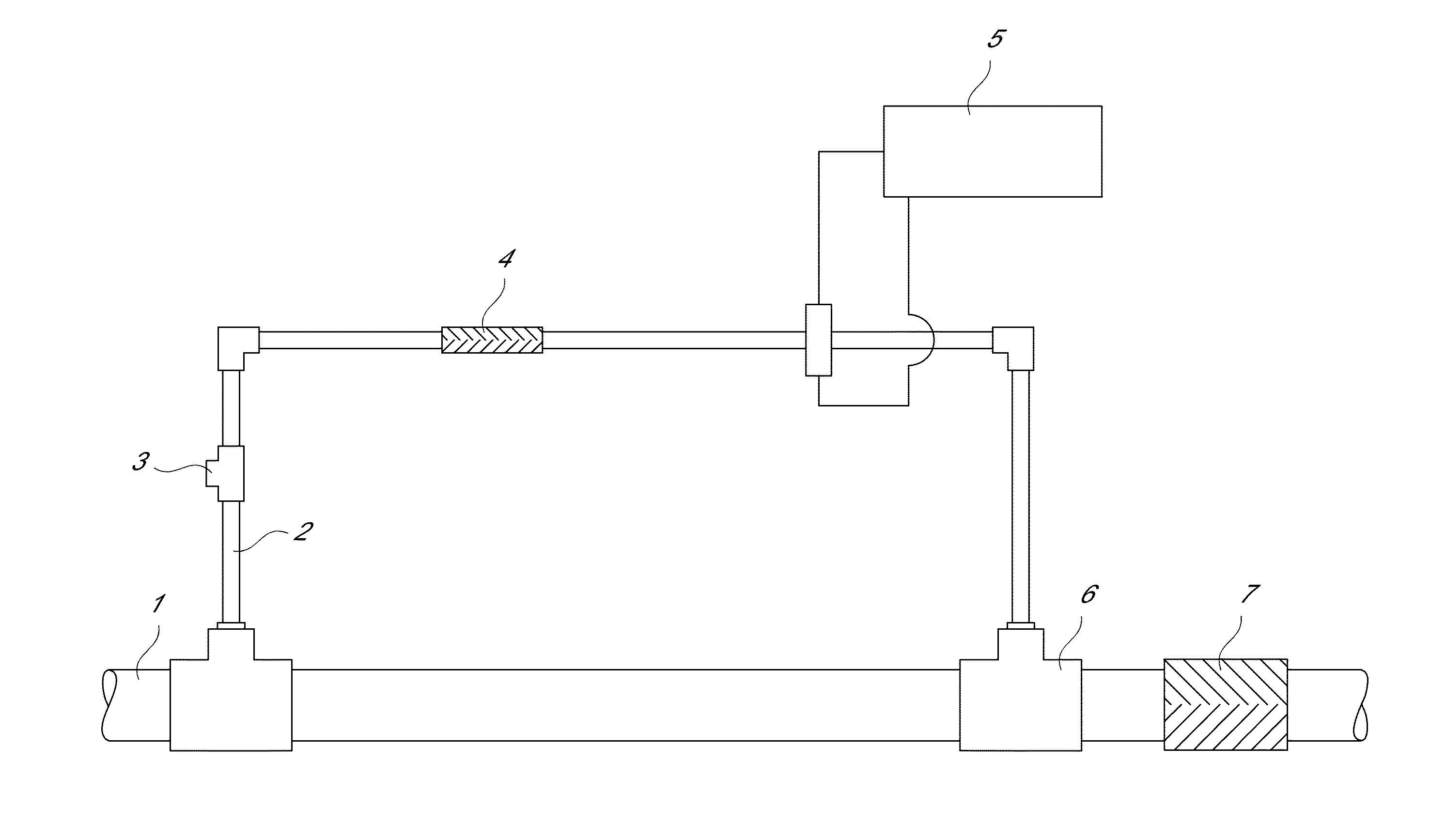
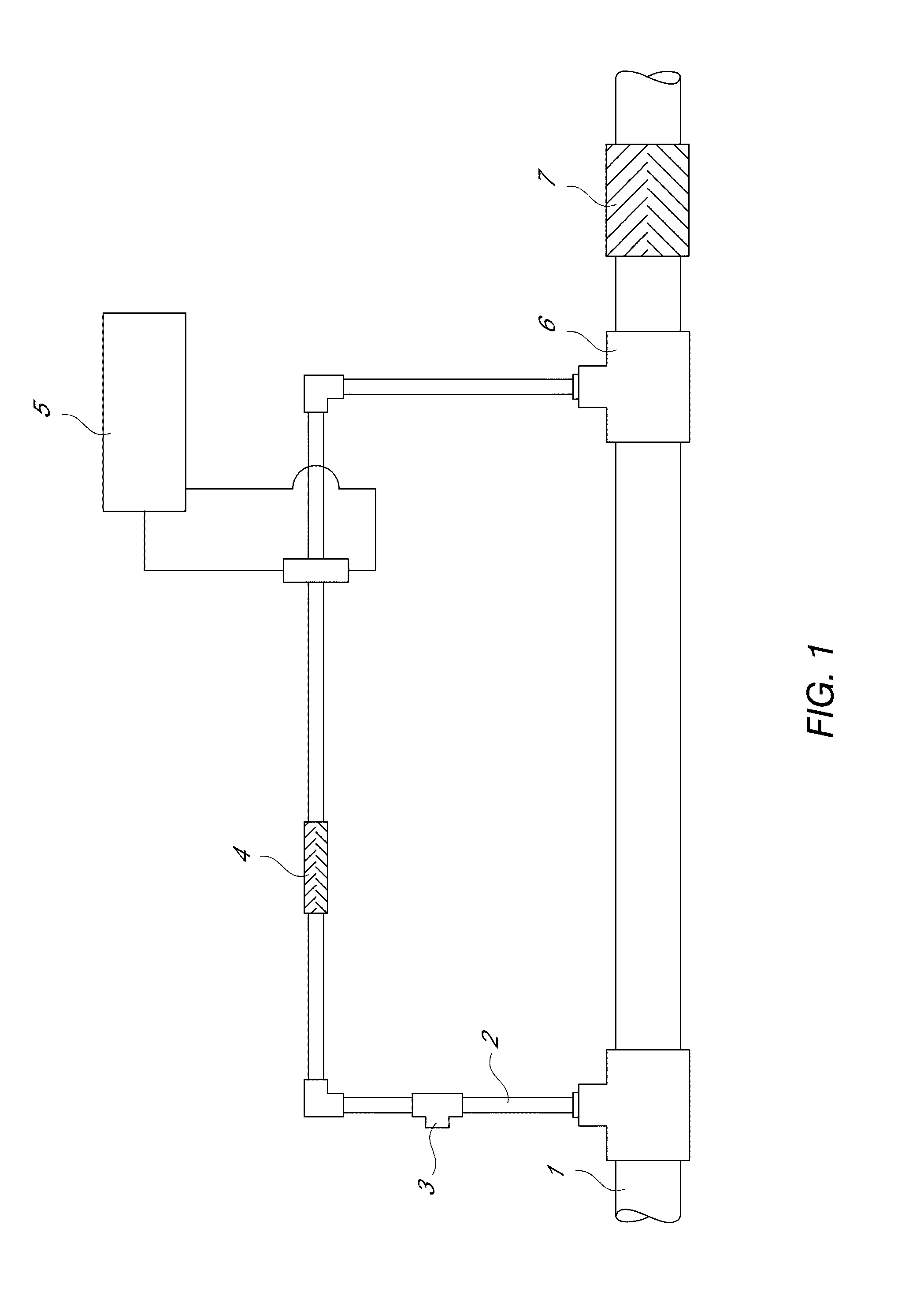
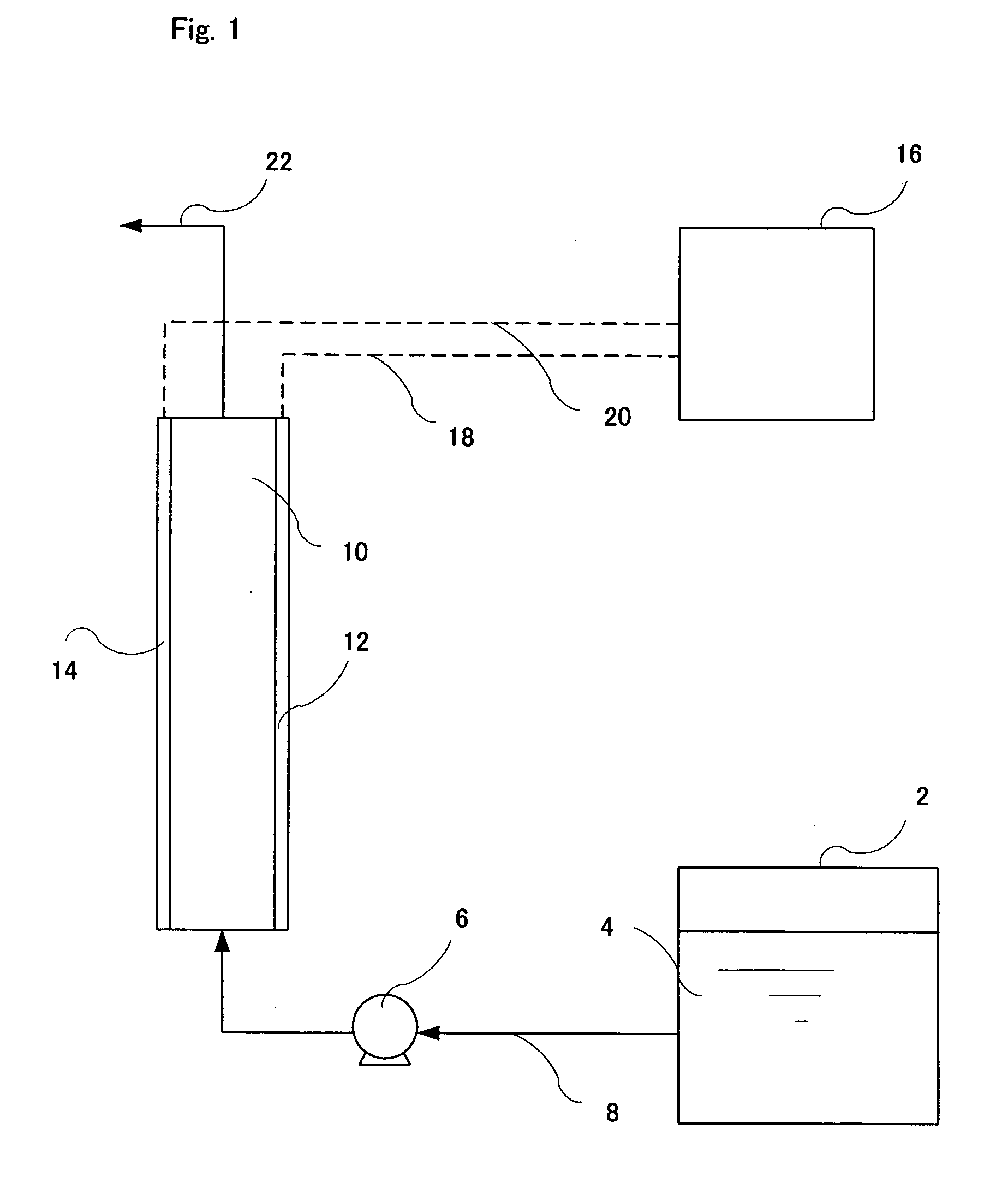
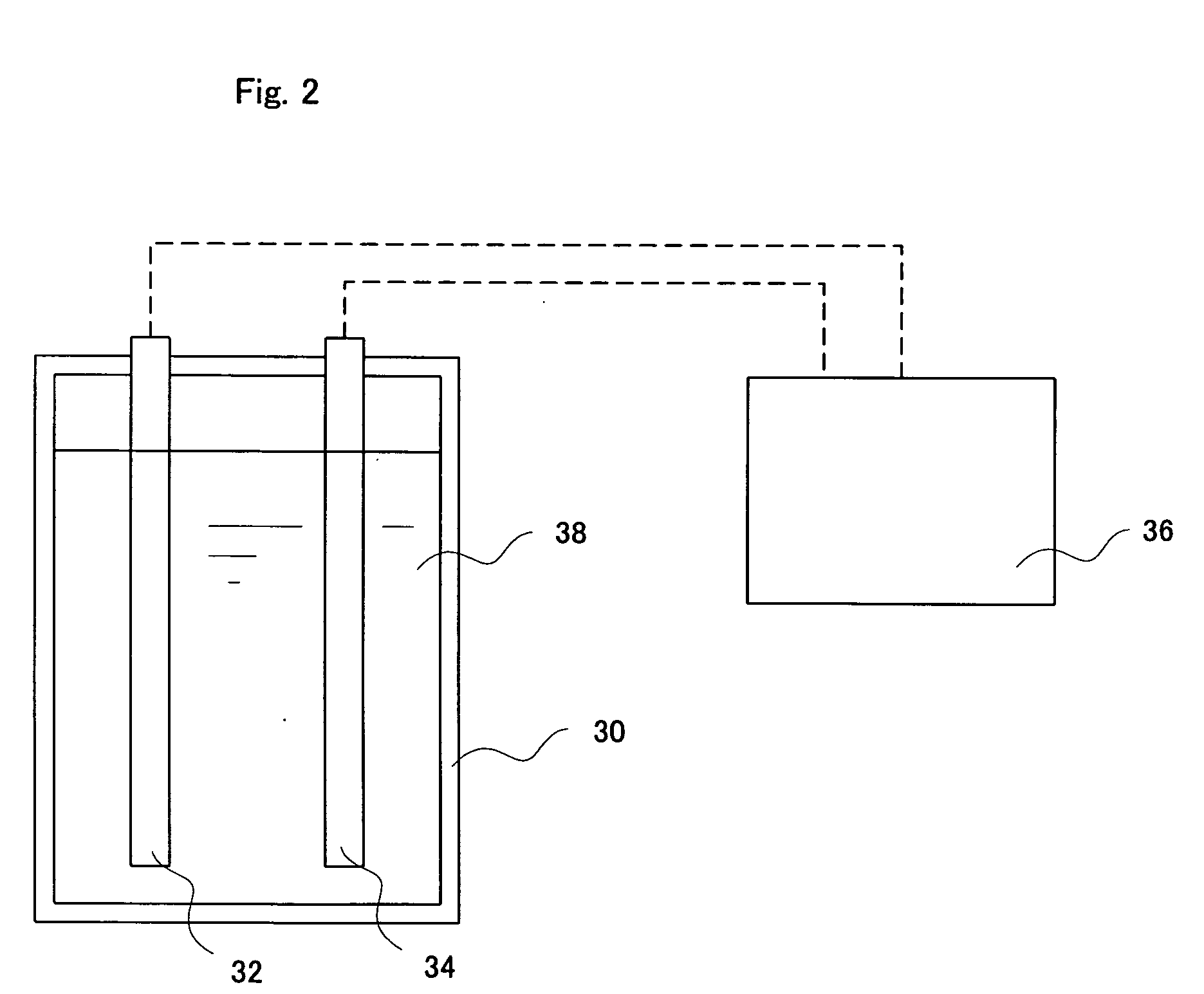
InactiveUS20130284678A1Increase and decrease demandLow in DOWater treatment parameter controlAnalysis using chemical indicatorsEngineeringVolume rate
An apparatus and methods for measuring the concentration of an additive are disclosed. The apparatus comprises a treatment stream (1) and a dosing stream (2). An additive is added to the dosing stream using a metering device (3). In some embodiments, the dosing stream is mixed after adding the additive using a first mixing device (4). Downstream from the metering device and the mixing device, the concentration of the additive in the dosing stream is measured using a monitor flow cell (5). In some embodiments, the dosing stream and treatment stream are combined (6) and mixed using a second mixing device (7). The concentration of the additive in the treatment stream can be calculated as a function of the volumetric flow rate ratio of the dosing stream to the treatment stream and the measured concentration of the additive in the dosing stream.
Owner:FERRATE TREATMENT TECH
Magnetic recording medium
ActiveUS7022424B2Improve featuresLow in DOMagnetic materials for record carriersRecord information storageX-rayNon magnetic
A magnetic recording medium comprising, in this order, a nonmagnetic support, a lower layer containing nonmagnetic powder and a binder, and a magnetic layer containing ferromagnetic powder and a binder, wherein N / Fe of the magnetic layer measured with a fluorescent X-ray apparatus is from 0.5 to 1.9 wt %, and a number of concavities having a depth of 5 to 10 nm on a surface of the magnetic layer is from 20 to 100 / 100 μm2.
Owner:FUJIFILM CORP +1
36-Des(3-Methoxy-4-Hydroxycyclohexyl) 36-(3-Hydroxycycloheptyl) Derivatives of Rapamycin for the Treatment of Cancer and Other Disorders
InactiveUS20090209572A1Solution stableResist attackBiocideOrganic chemistryImmunologic disordersFibrosis
The present invention relates to novel 36-des(3-methoxy-4-hydroxycyclohexyl)-36-(3-hydroxycycloheptyl)rapamycin derivatives, methods for their production, and uses thereof. In a further aspect the present invention provides for the use of these 36-des(3-methoxy-4-hydroxycyclohexyl)-36-(3-hydroxycycloheptyl)rapamycin derivatives in the treatment of cancer and / or B-cell malignancies, the induction or maintenance of immunosuppression, the treatment of transplantation rejection, graft vs. host disease, autoimmune disorders, diseases of inflammation, vascular disease and fibrotic diseases, the stimulation of neuronal regeneration or the treatment of fungal infections.
Owner:BIOTICA TECH
Methods and compositions for optimization of oxygen transport by cell-free systems
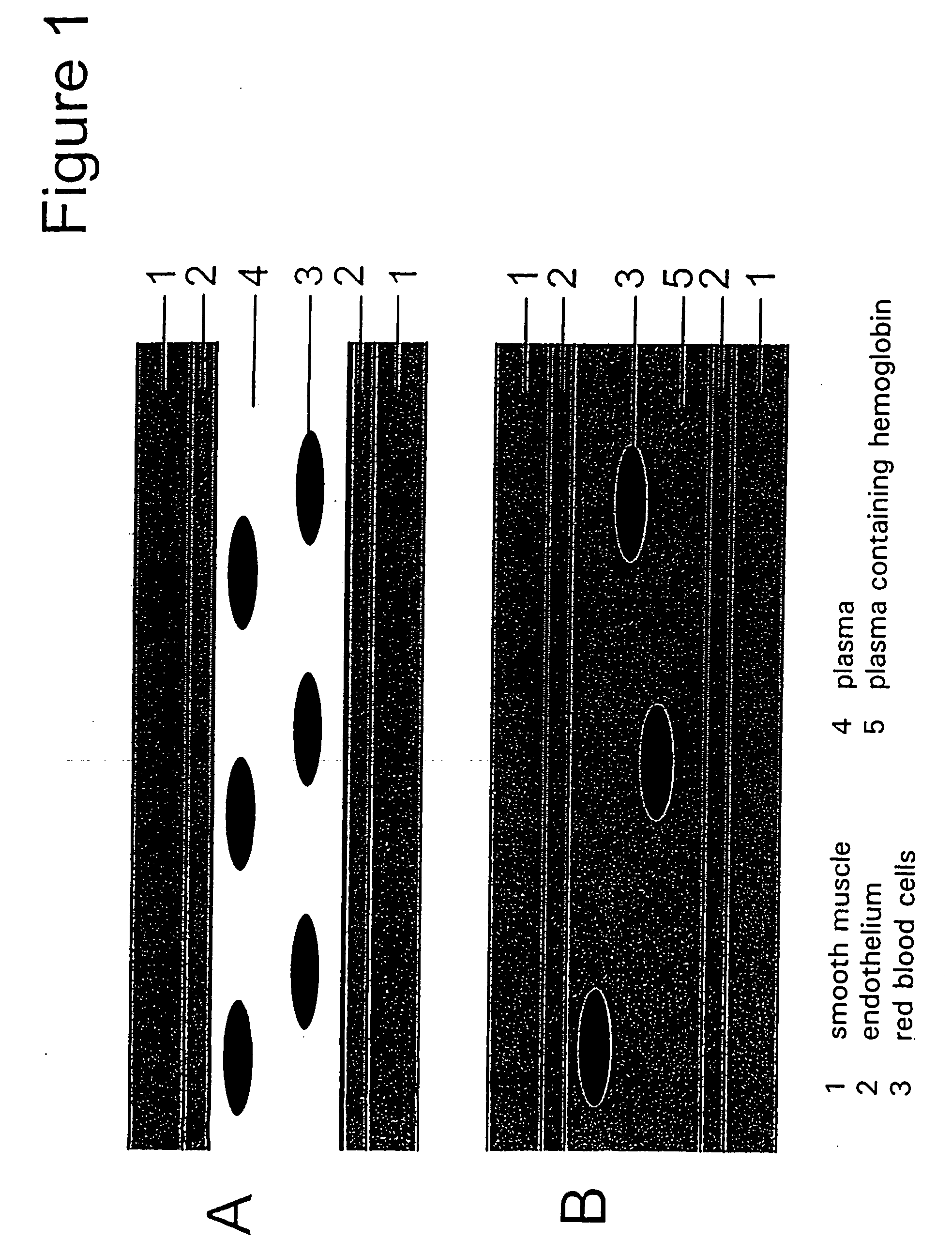
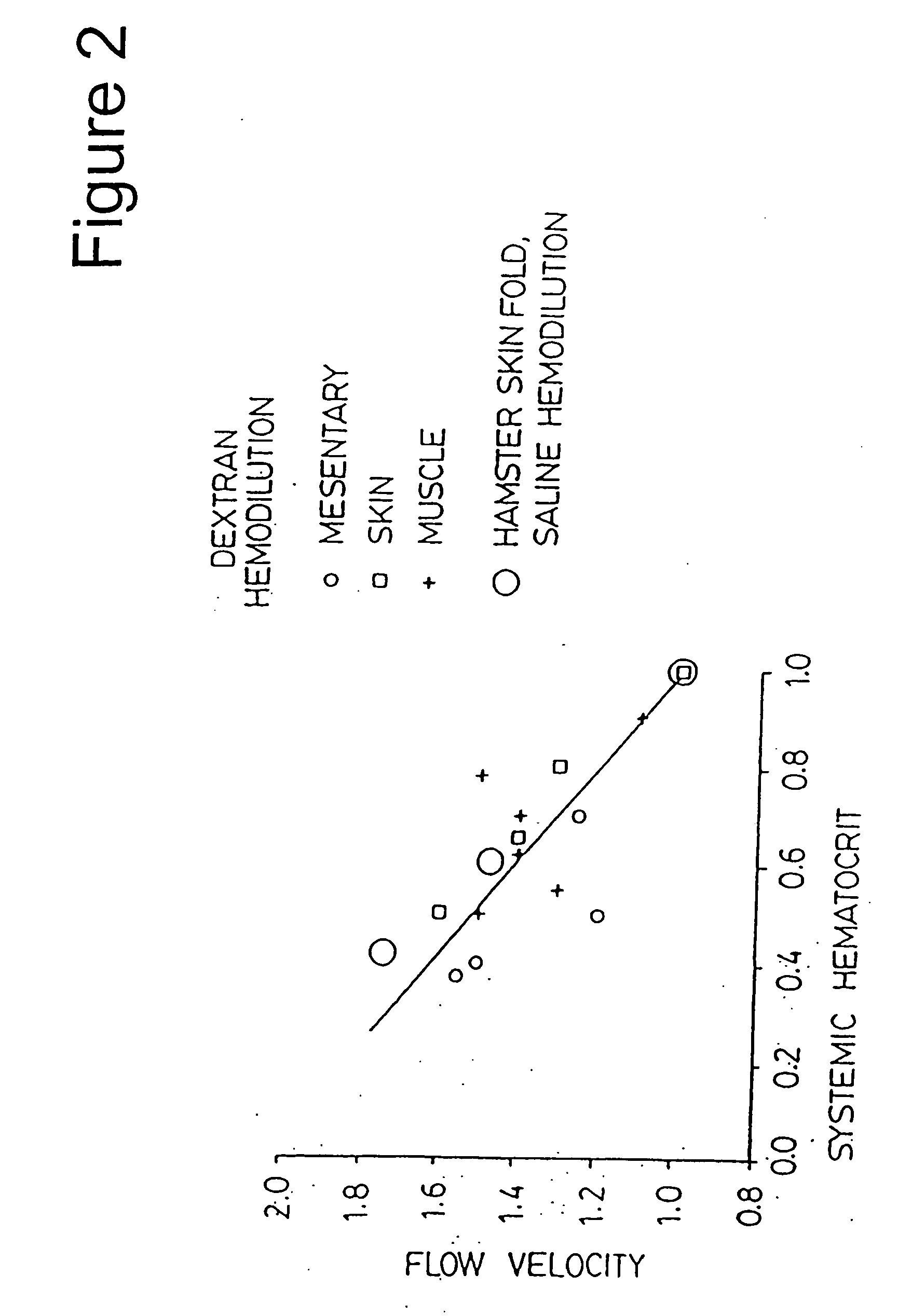
InactiveUS20050239686A1Limit on characteristicLow in DOOrganic active ingredientsPeptide/protein ingredientsCell freePlasma expander
Compositions, and methods of use thereof, for use as blood substitute products comprise aqueous mixtures of oxygen-carrying and non-oxygen carrying plasma expanders and methods for the use thereof. The oxygen-carrying component may consist of any hemoglobin-based oxygen carrier, while the non-oxygen carrying plasma expander my consist of any suitable diluent.
Owner:WINSLOW ROBERT M
Method and Composition to Increase Radiation-Induced Tumor Therapeutic Effects
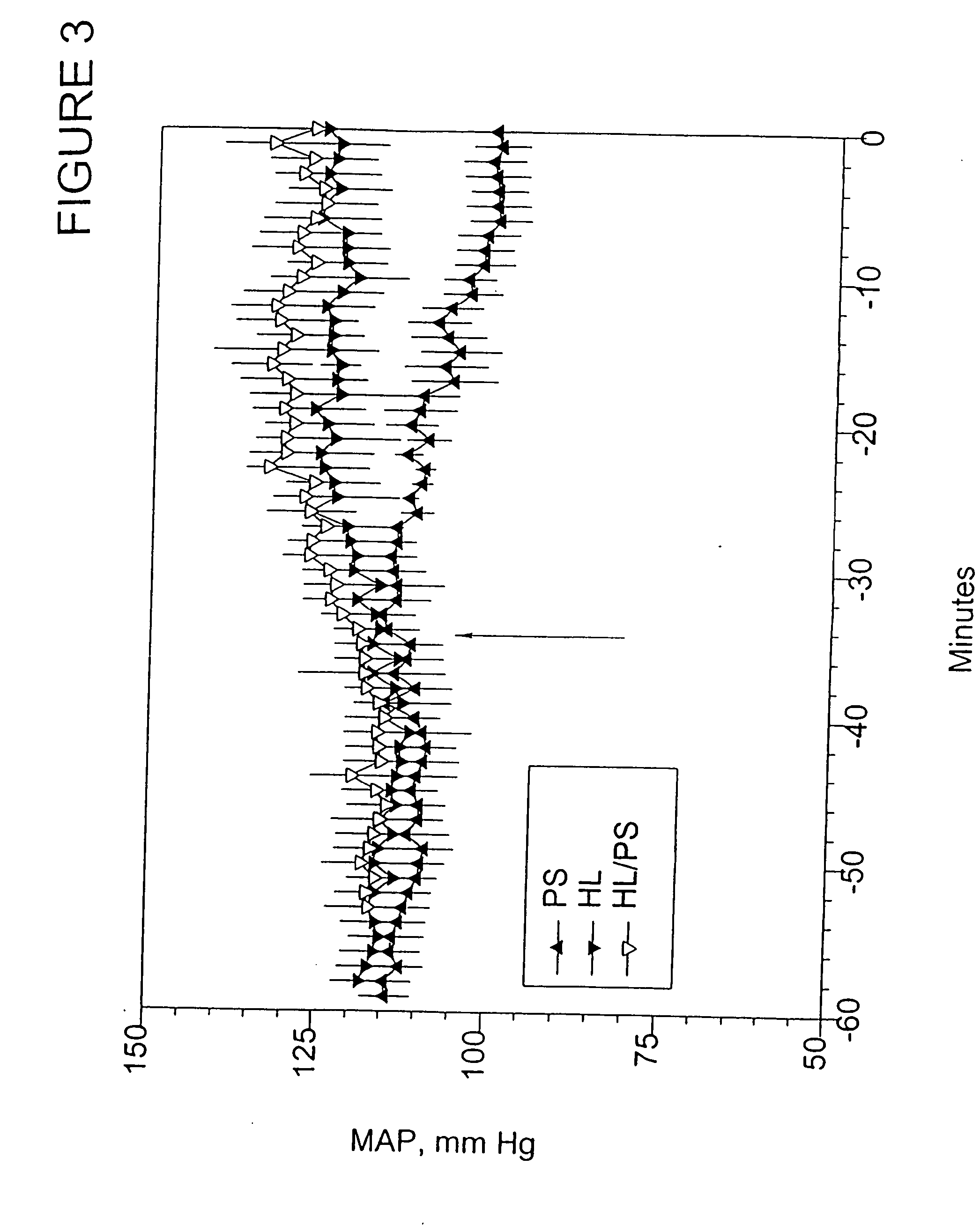
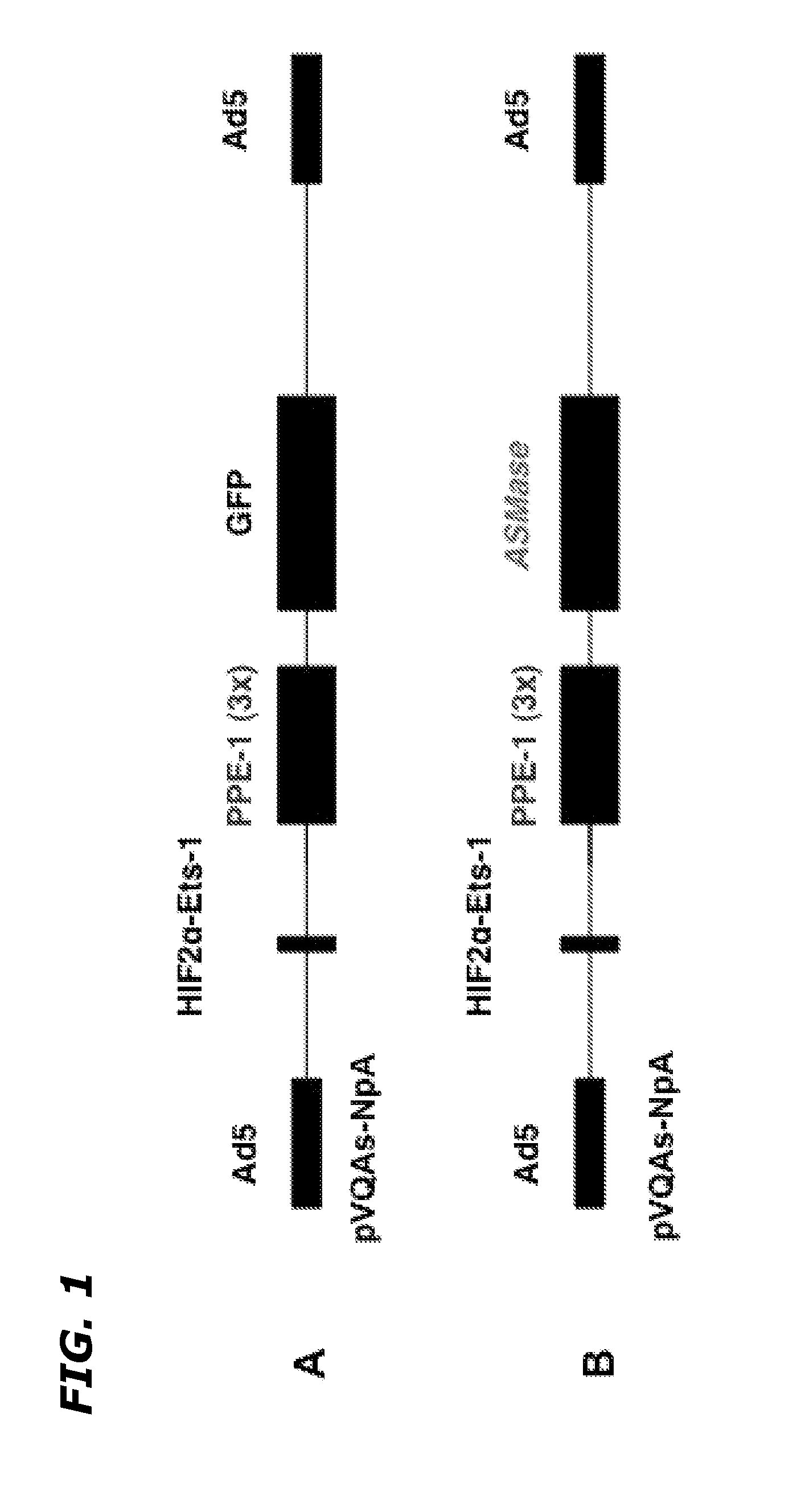
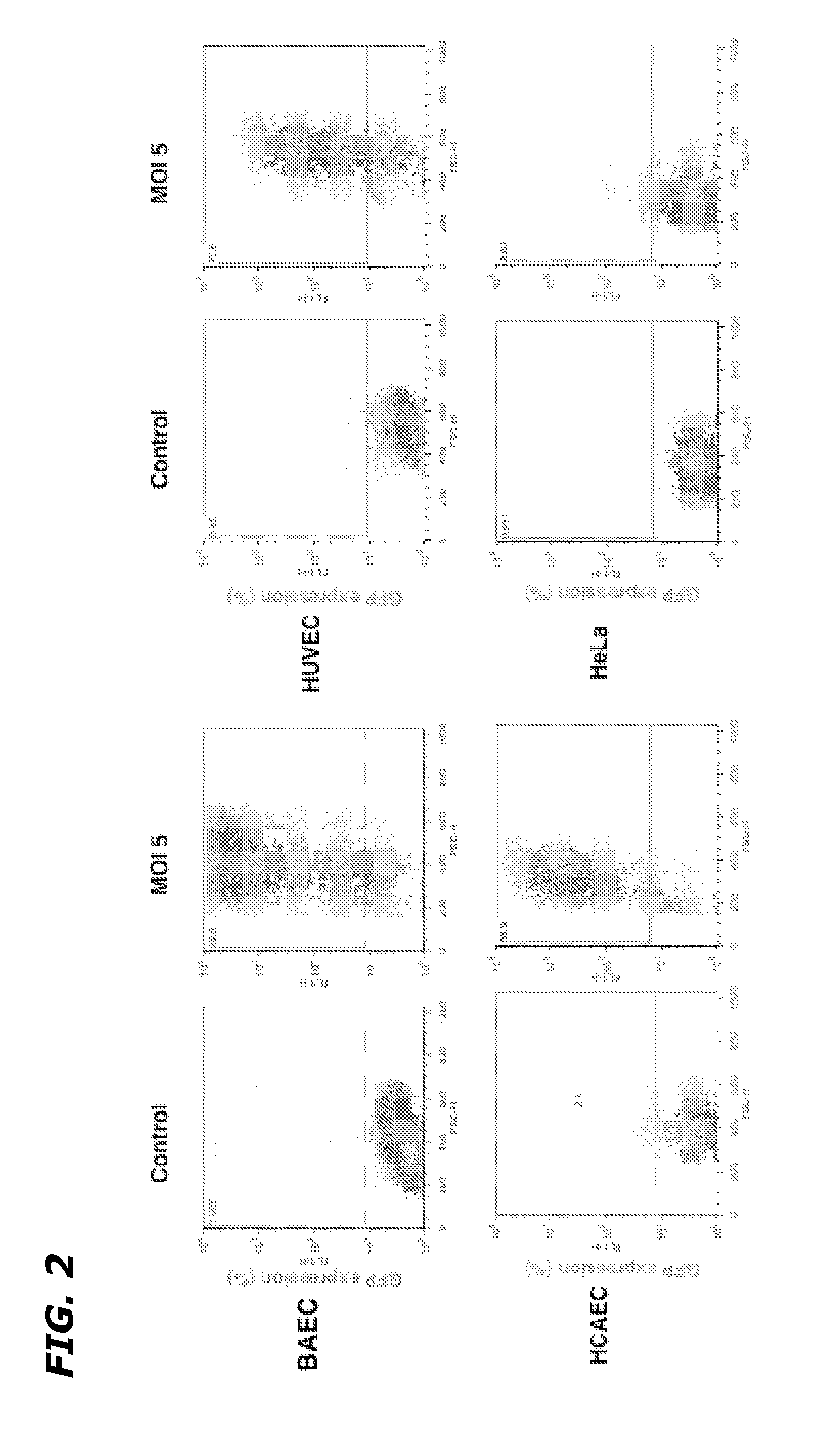
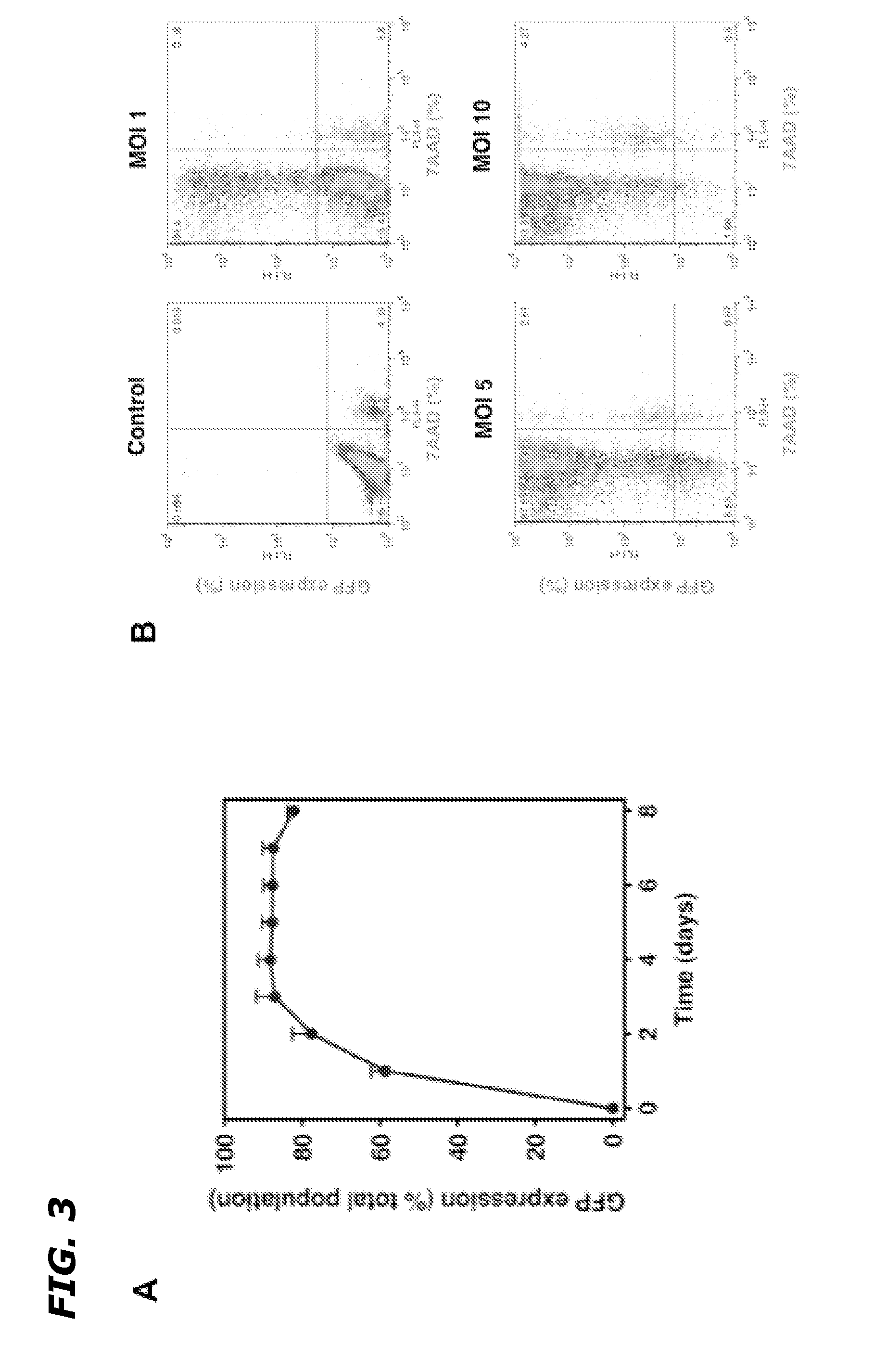
InactiveUS20120252883A1Improve the protective effectReduced effectivenessOrganic active ingredientsPeptide/protein ingredientsSide effectDNA construct
Disclosed herein are methods and compositions for treating cancer by increasing radiation-induced damage to cancer without increasing radiation-induced side effects by increasing secretory ASMase levels specifically in tumor endothelium, and inducing apoptosis of tumor endothelial cells by treating the tumor with radiation. ASMase levels are increased in tumor endothelium by administration of a recombinant DNA construct comprising a region coding for a functional ASMase linked to particular transcriptional regulatory sequences that confer tissue-specific expression of ASMase.
Owner:VASCULAR BIOGENICS +1
Augmented Bypass Catheter
ActiveUS20190217051A1Relieve strainDissolve clotUltrasound therapyBalloon catheterLysisMedical treatment
An innovative medical device that permits rapid, minimally invasive restoration of blood flow across a vascular blockage. A method employing said device, allowing for lysis or removal of said blockage. Said removal of said blockage is facilitated by either or both mechanical and energy-emission maceration. Said device creates a temporary bypass using longitudinal structure configured for insertion into the blood vessel and adapted to deliver a side hole to a target area. The side hole defines a distal first segment and a proximal second segment with a lumen to allow blood flow therethrough to the distal end hole. In an alternate embodiment, a slide-able outer sheath can cover the side hole to permit reversal of blood flow from the distal end hole to a proximal end hole located outside a patient's body by means of an aspiration controller. Alternate embodiments include an optional anchoring balloon, a macerating stent or wires, perforations for fluid delivery, and a backflow valve.
Owner:WALZMAN DANIEL EZRA
Salmonella-based vectors for cancer immunotherapy targeting wilms' tumor gene WT1
ActiveUS9920297B2Attenuated <i>SalmonellaRisk minimizationTumor rejection antigen precursorsBacteriaSalmonella SenftenbergRecombinant DNA
The present invention relates to an attenuated mutant strain of Salmonella comprising a recombinant DNA molecule encoding Wilms' tumor Protein 1. In particular, the present invention relates to the use of said attenuated mutant strain of Salmonella in cancer immunotherapy.
Owner:VAXIMM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![[[2-(Amino-3,4-dioxo-1-cyclobuten-1-yl)amino]alkyl]-acid derivatives for the treatment of pain [[2-(Amino-3,4-dioxo-1-cyclobuten-1-yl)amino]alkyl]-acid derivatives for the treatment of pain](https://images-eureka.patsnap.com/patent_img/ff0e11a9-070d-40fc-9119-9f3dcf53db3e/US20030114444A1-20030619-C00001.png)
![[[2-(Amino-3,4-dioxo-1-cyclobuten-1-yl)amino]alkyl]-acid derivatives for the treatment of pain [[2-(Amino-3,4-dioxo-1-cyclobuten-1-yl)amino]alkyl]-acid derivatives for the treatment of pain](https://images-eureka.patsnap.com/patent_img/ff0e11a9-070d-40fc-9119-9f3dcf53db3e/US20030114444A1-20030619-C00002.png)
![[[2-(Amino-3,4-dioxo-1-cyclobuten-1-yl)amino]alkyl]-acid derivatives for the treatment of pain [[2-(Amino-3,4-dioxo-1-cyclobuten-1-yl)amino]alkyl]-acid derivatives for the treatment of pain](https://images-eureka.patsnap.com/patent_img/ff0e11a9-070d-40fc-9119-9f3dcf53db3e/US20030114444A1-20030619-C00003.png)