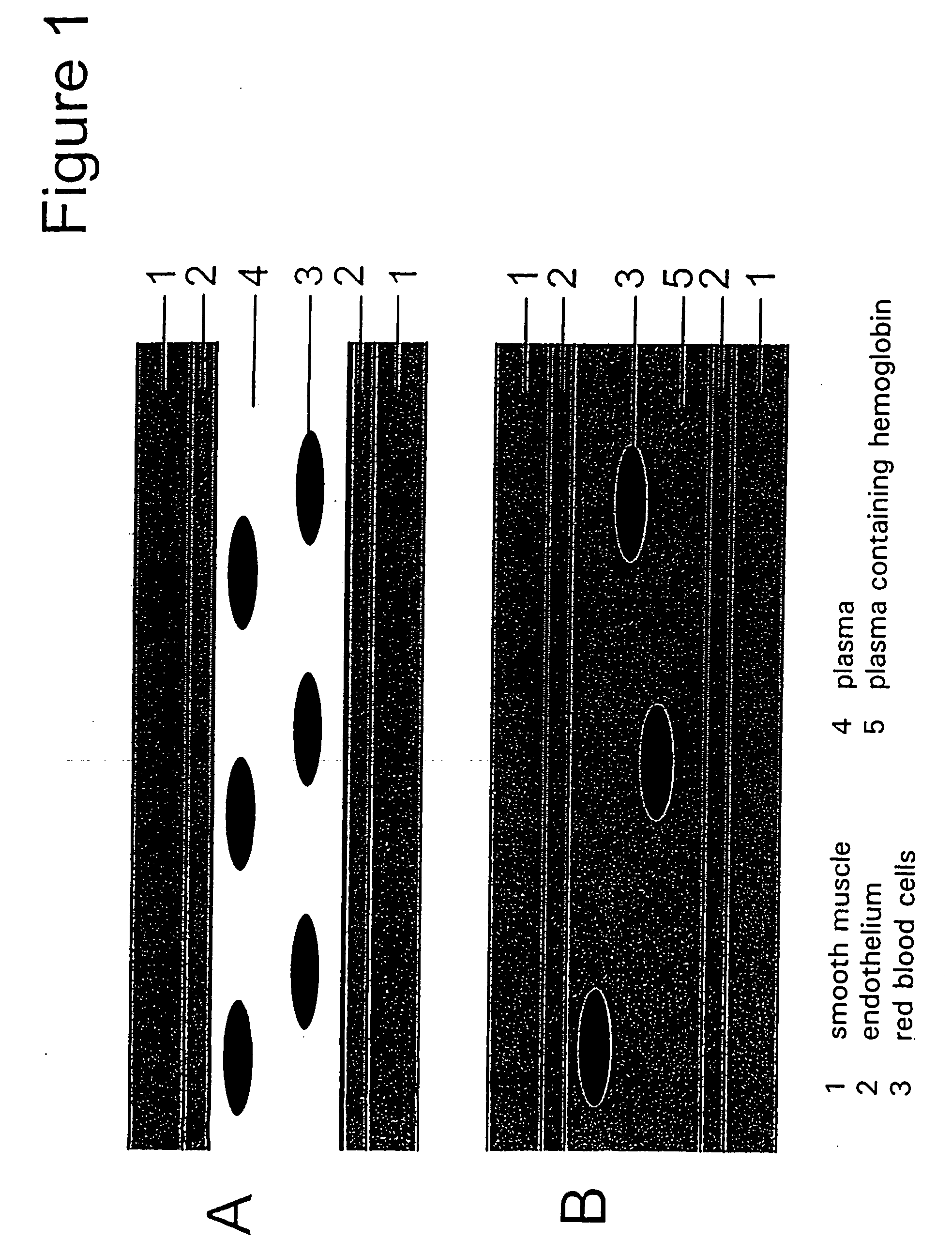
Methods and compositions for optimization of oxygen transport by cell-free systems
a cell-free system and oxygen transport technology, applied in the field of blood products, can solve the problems of affecting the efficiency of oxygen transporting plasma expanders, and whose production is tedious and expensive, and achieves the effects of marginal efficacy, and reducing the number of oxygen transporters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
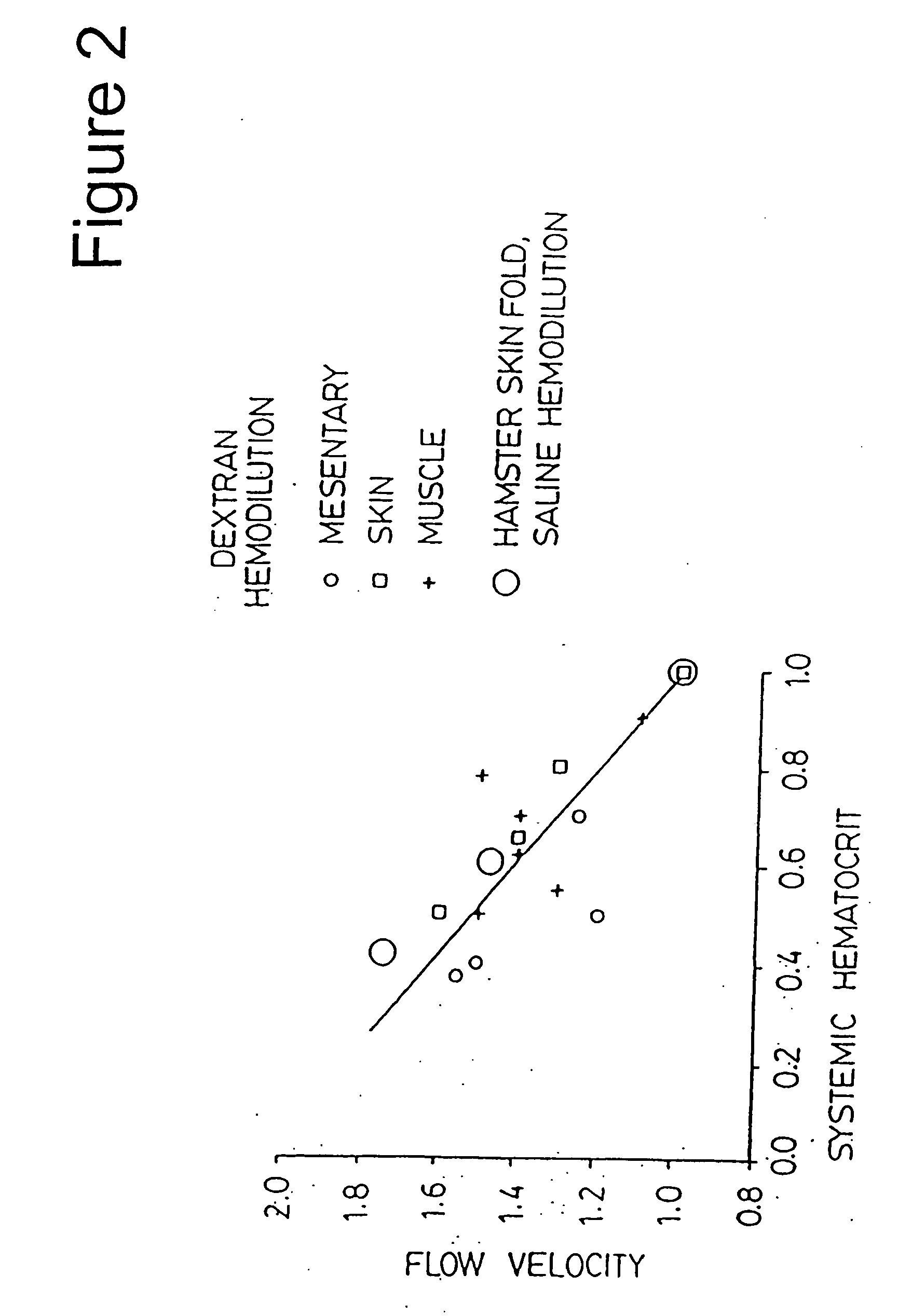
Blood Flow and Hematocrit During Colloid and Saline Hemodilution
[0235] The experiments of this example were directed at determining the effect of decreasing hematocrit, as a result of hemodilution, on blood flow velocity. The experiments of this example were conducted on hamsters using dextran 70 and saline.
[0236] The general experimental procedures (e.g., General Experimental Protocol and Capillary Red Blood Cell Velocity) described above were performed. FIG. 2 depicts a plot of flow velocity in the microcirculation as a function of hematocrit reductions with dextran hemodilution and saline hemodilution. The following designations are used in FIG. 2: i) dextran hemodilution: small circle=mesentery; square=skin; plus sign=muscle; and ii) saline hemodilution: large circle=skin fold. The results indicate that blood flow, as evidenced by the velocity of blood in the vessel of the microcirculation, increases as blood is diluted. The increase is linearly related to the decrease of hema...
example 2
pO2 Distribution During Dextran 70 and Hemolink® Hemodilution
[0238] The experiments of this example were directed at determining the effect of hemodilution on pO2 in the microcirculation by the phosphorescence decay method described above.
Dextran 70 Hemodilution
[0239] Measurements of pO2 were made in 50 μm arterioles and the tissue surrounding those arterioles. The results were as follows: arteriole pO2 (pO2.A)=53 mm Hg; tissue pO2 (pO2.T)=21 mm Hg. The following equation may then be utilized to calculate KA*, the constant representing the difference in the decrease in the oxygen partial pressure between i) the arterioles and the tissues and ii) the central arteries and the tissues:
KA*=In[(pO2.A−pO2.T) / (pO2.a−pO2.T)]
where pO2.a is the oxygen tension in a central artery. If one assumes a pO2.a=100 mm Hg, then KA*=In [(53-21) / (100-21)]=−0.90.
[0240] Table 5 sets forth previously obtained (by the present inventors) pO2 values for various hematocrit (α) levels with dextran 70 hemod...
example 3
Tissue Oxygenation Resulting from Hemodilution with 50% Hemolink® / 50% Dextran 70
[0247] The experiments of this example are directed at determining the adequacy of tissue oxygenation following administration of a mixture of Hemolink® and dextran 70.
[0248] A mixture of 50% Hemolink® and 50% dextran 70 was prepared, and tissue oxygenation was determined at hematocrit levels of 60% and 40% of baseline levels. Hemoglobin concentration in the resulting mixture was measured directly by spectrophotometry. In addition, the number of RBCs and the amount of Hemolink® were measured directly in blood samples. Though testing was initiated using four animals, only two animals satisfied all criteria for inclusion in an experimental run; the results for the two animals are set forth in Table 7.
TABLE 7pO2.A Meas.Wall Grad.pO2.THtc / αγγ / αpO2.Amm Hgmm Hgmm Hg0.6 / 0.680.640.86550.4 / 0.540.761.4143512715
[0249] When the data in Table 7 is compared with that derived from use of Hemolink® alone (see Table ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| oncotic pressure | aaaaa | aaaaa |
| oncotic pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com