Method for simultaneously detecting neoantigen immunogenicity and neoantigen specificity TCR (T cell receptor)
An antigen immunization and specific technology, applied in the field of molecular biology and cellular immunology, can solve the problems of high cost of tetramer, difficult and expensive preparation of tetramer, and achieve high test result accuracy and test throughput. Unrestricted, time-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
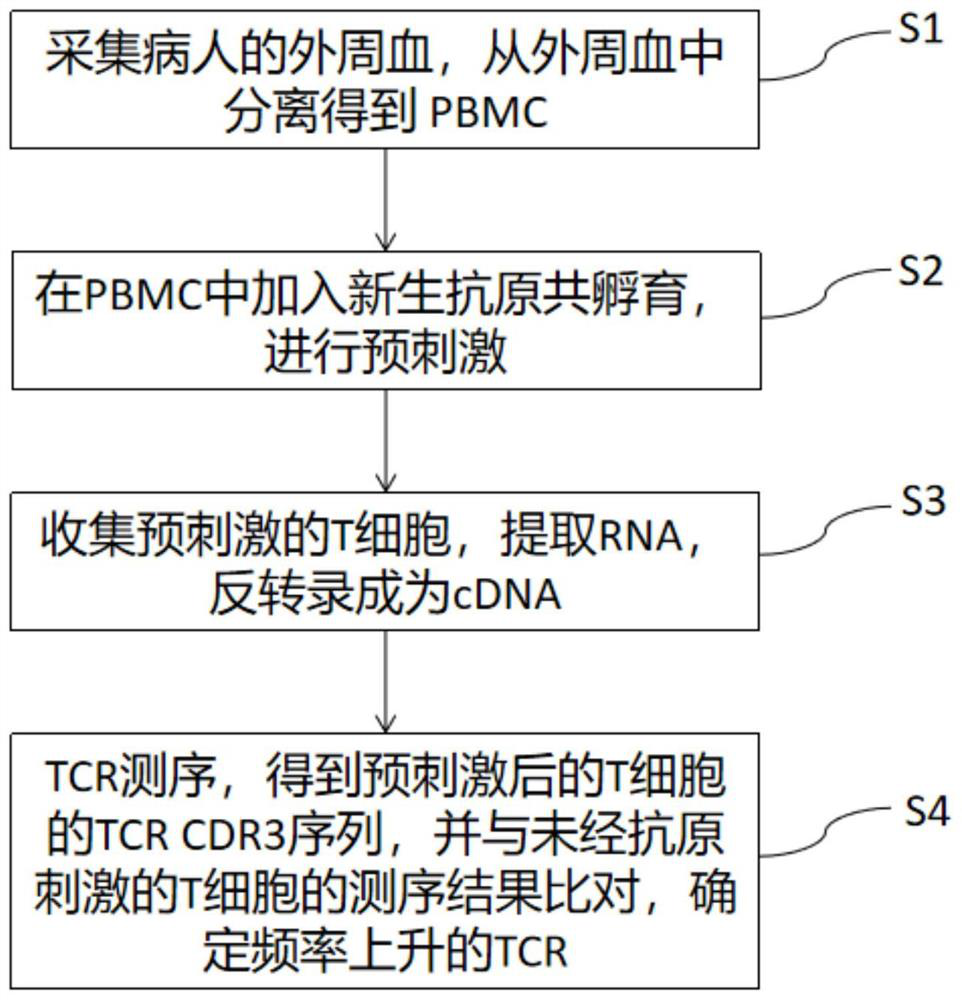
[0041] This example provides a method for simultaneously detecting neoantigen immunogenicity and neoantigen-specific TCR, such as figure 1 As shown, the method includes the following steps:
[0042] S1. Collect the peripheral blood of the patient, and separate peripheral blood mononuclear cells (PBMC) from the peripheral blood.
[0043] First, take 20ml of peripheral blood from the patient, use lymphocyte separation medium, and obtain PBMC by density gradient centrifugation, add serum-free AIM-V medium to it, and resuspend the PBMC to 1×10 6 cells / ml, and spread PBMCs in 18 wells of a 24-well plate according to 1ml / well.
[0044] S2. Add neoantigens to PBMCs to start co-incubation for pre-stimulation, and collect pre-stimulated cells on the tenth day of incubation.
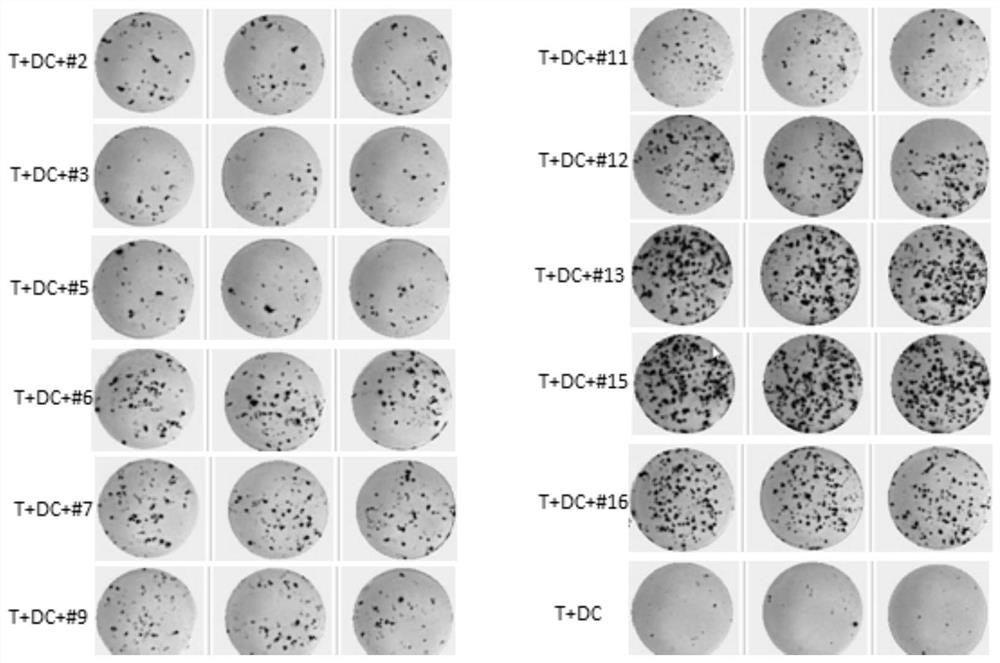
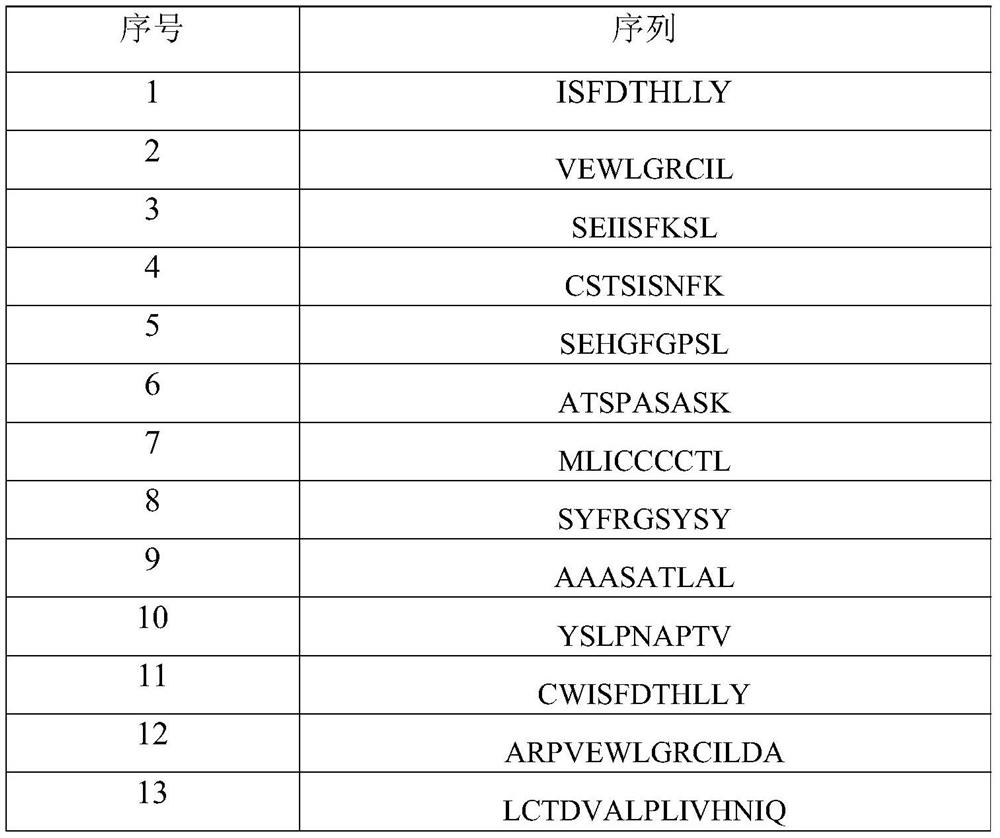
[0045] Add 17 kinds of neoantigens to the 17 wells covered with PBMC and medium for co-incubation, and no neoantigens are added to the 18th well. 2 cultured in an incubator for 10 days, and the pre-stimulated c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com