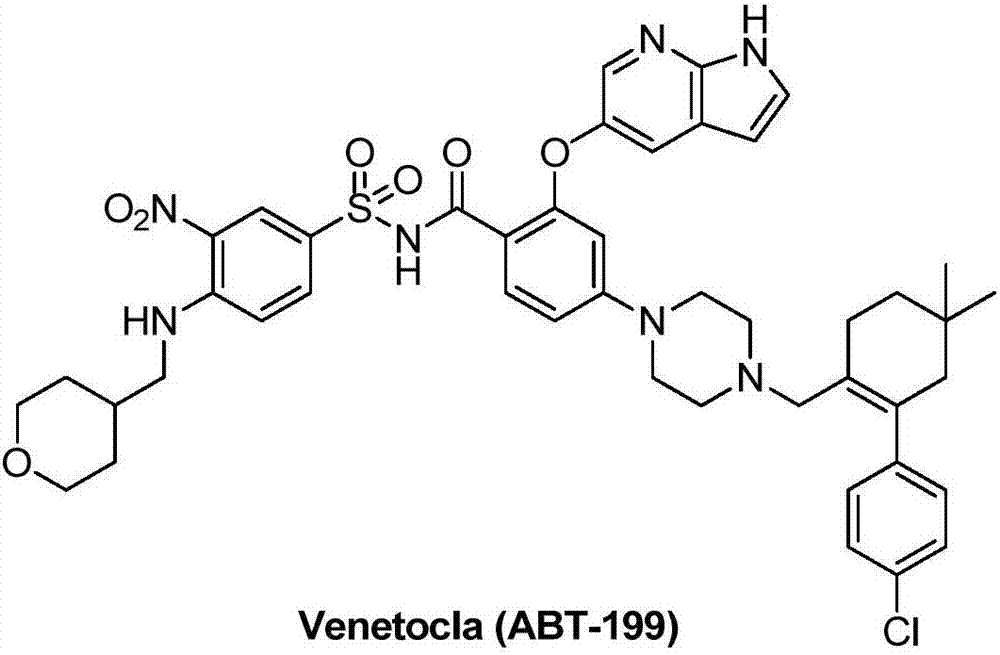
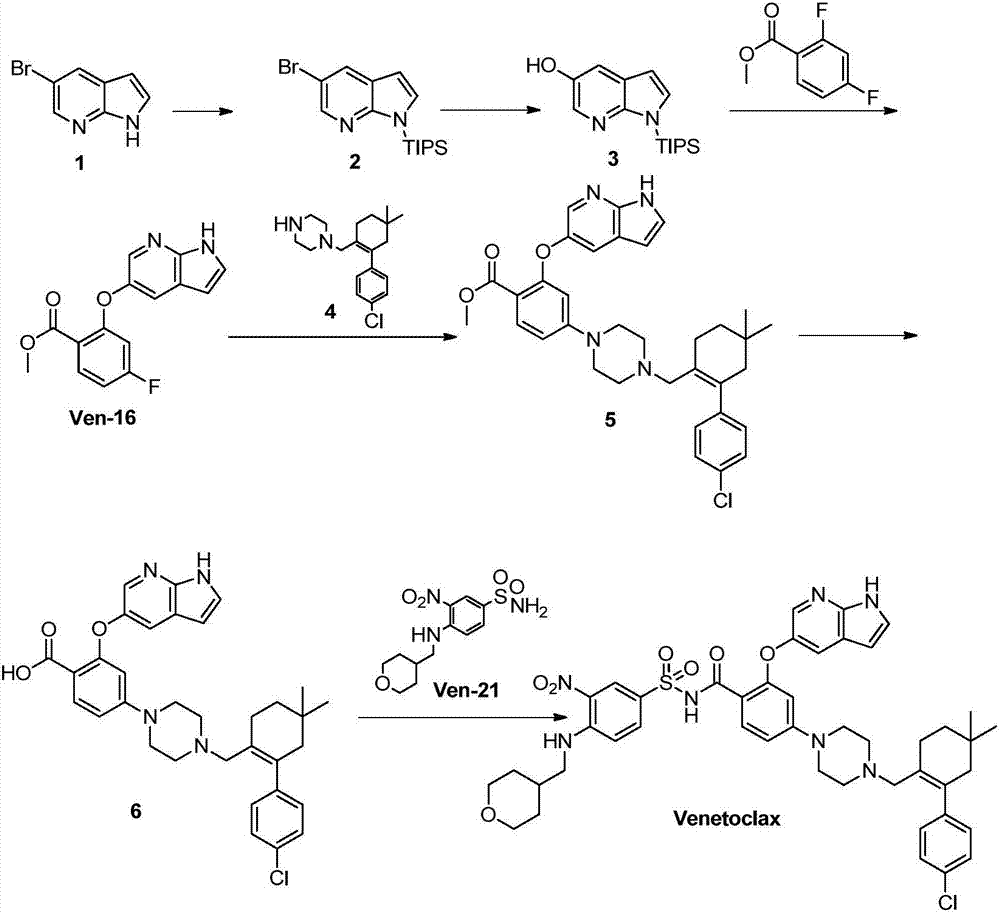
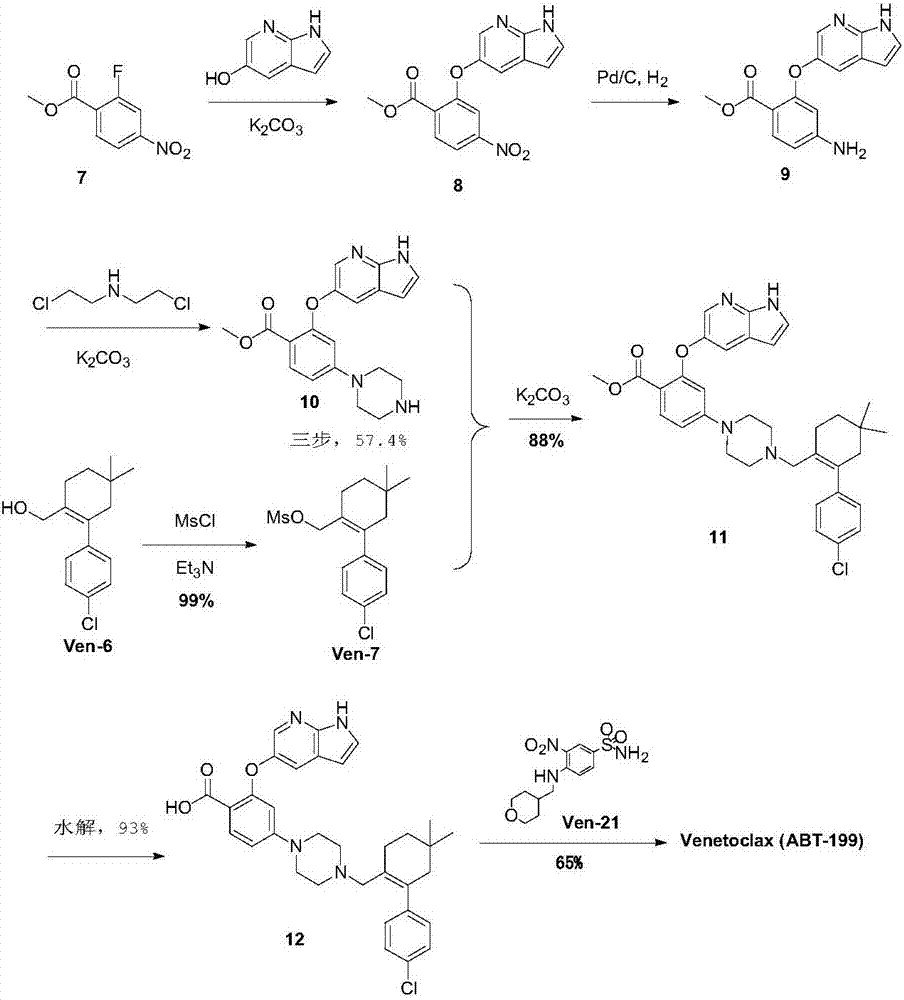
Synthesis method for BCL-2 inhibitor Venetoclax
A synthetic method, BCL-2 technology, applied in the direction of organic chemistry, etc., can solve the problems of no commercial large-scale supply, no industrial production, difficult to scale up the reaction, etc., achieve important industrial application value, low synthesis cost, high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1Ven-16 synthesis:
[0034] Add 100g of 5-hydroxy-7-azaindole (746mmol), 141g of methyl 2,4-difluorobenzoate (821mmol), 237g of potassium phosphate (1.12mol) and 500mL of diethylene glycol dimethyl to the three-necked flask Ether, reacted at 110°C for 24h (the reaction of 5-hydroxy-7-azaindole was completely monitored by HPLC). The reaction solution was concentrated to dryness, stirred by adding 2L of ethyl acetate and 2L of water, the organic phase was separated, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain a crude product. The crude product was heated to reflux with 1260mL ethyl acetate until it was cleared, slowly added dropwise 1260mL of petroleum ether, and the dripping was completed after 1h, and continued to stir under reflux for 1h, slowly cooled to 25°C, filtered, and dried to obtain 163g of a light white solid. The rate is 76.5%. HPLC purity 98%.
[0035] The reaction formula is as follows:
[0036]
Embodiment 2Ve
[0037] Embodiment 2 Ven-17 synthesis:
[0038]Add 30g Ven-16 (104.9mmol), 600mL DMSO, 23.4g (125.9mmol) N-Boc-piperazine and 35.9g (157.4mmol) dipotassium hydrogen phosphate to the reaction flask, raise the temperature to 120°C and react for 24h (monitored by TLC Ven-16 reaction is complete), add 900mL ethyl acetate and 900mL water and stir for 30min, then separate the liquids, wash the organic phase with 2×900mL water, dry over anhydrous sodium sulfate, and concentrate to dryness to obtain 66g of light yellow solid. directly into the next reaction.
[0039] The reaction formula is as follows:
[0040]
Embodiment 3Ve
[0041] Embodiment 3 Ven-18 synthesis:
[0042] Add 40g Ven-17 (88mmol) and 400mL tetrahydrofuran to the reaction flask, add dropwise 500mL of 10% sodium hydroxide aqueous solution, heat up to 65°C for 24h, TLC monitors that the reaction of the raw materials is complete, cool to room temperature, add 400mL of methyl tert-butyl Stir the ether, separate the phase of methyl tert-butyl ether, adjust the pH to 4-5 with 10% hydrochloric acid solution, a solid precipitates, continue to stir for 1 h, filter, and dry at 45 ° C to obtain 39 g of white powdery solid, yield 100 %.
[0043] The reaction formula is as follows:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com