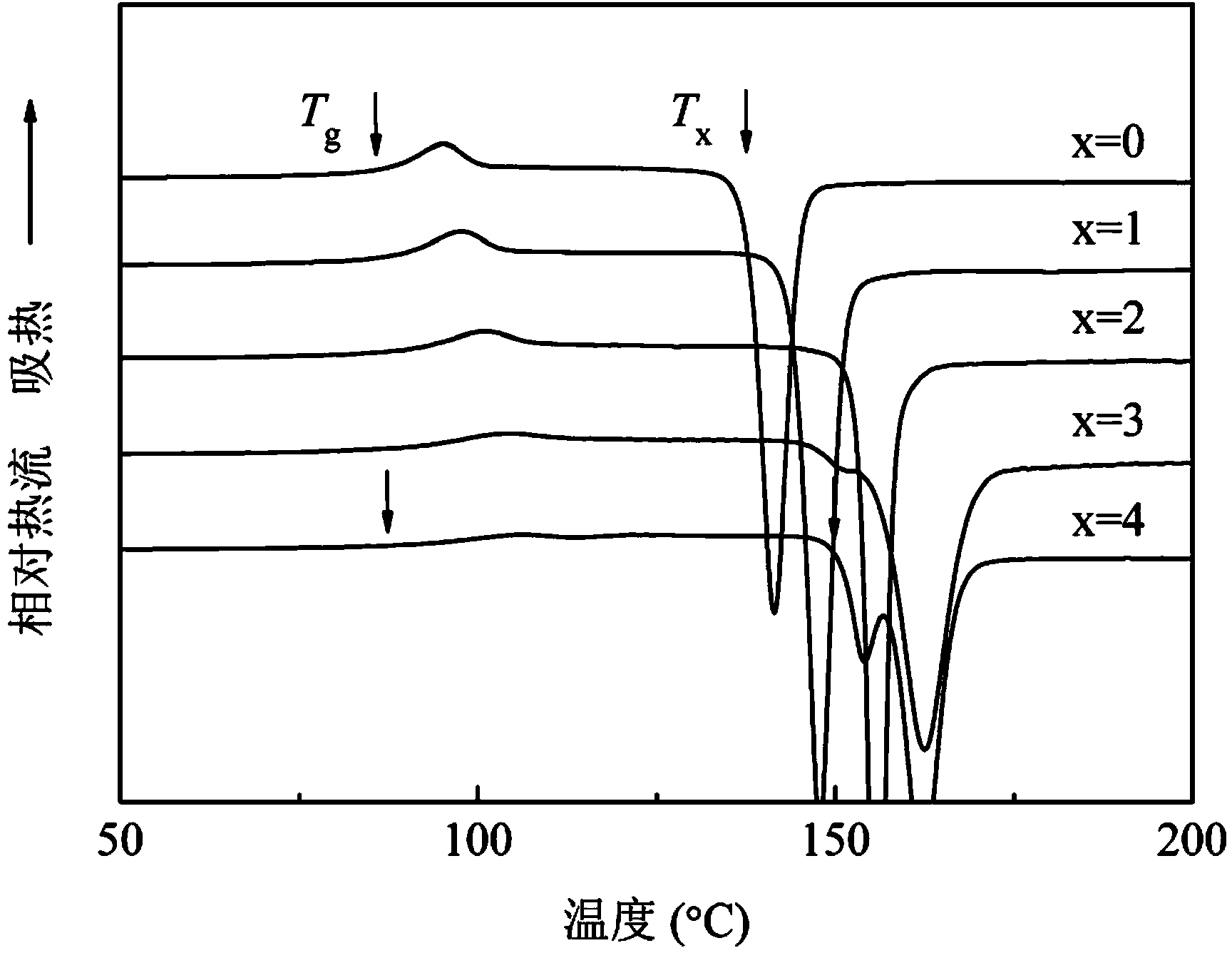
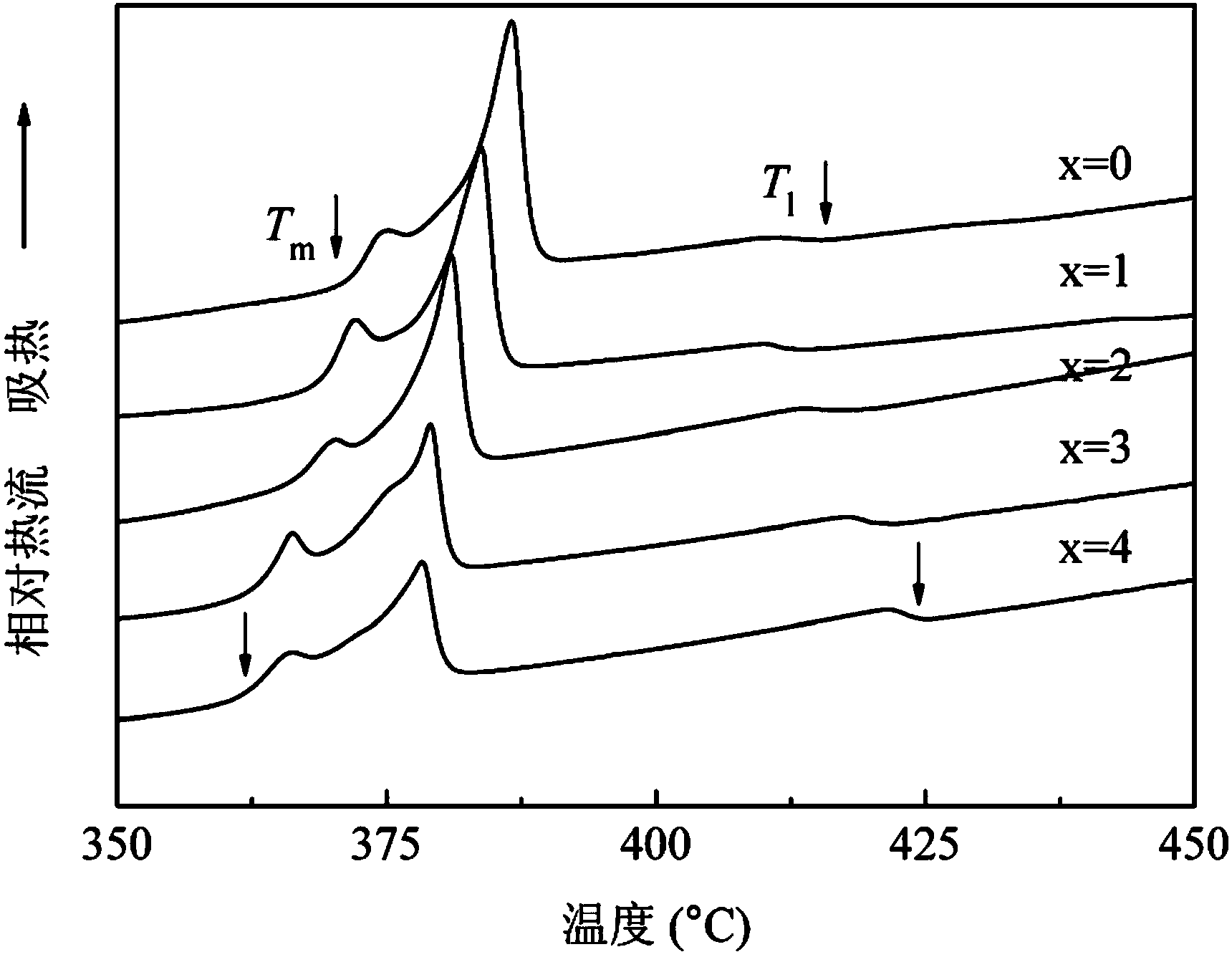
Patents
Literature
135results about How to "High glass forming ability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Al2O3-Y2O3-ZrO2/HfO2 materials, and methods of making and using the same
InactiveUS7507268B2Facilitates formation and homogeneityEliminates and minimizes heat transferPigmenting treatmentGlass drawing apparatusFiberThermal insulation
Al2O3—Y2O3—ZrO2 / HfO2 ceramics (including glasses, crystalline ceramics, and glass-ceramics) and methods of making the same. Ceramics according to the present invention can be made, formed as, or converted into glass beads, articles (e.g., plates), fibers, particles, and thin coatings. The particles and fibers are useful, for example, as thermal insulation, filler, or reinforcing material in composites (e.g., ceramic, metal, or polymeric matrix composites). The thin coatings can be useful, for example, as protective coatings in applications involving wear, as well as for thermal management. Certain ceramic particles according to the present invention can be are particularly useful as abrasive particles.
Owner:3M INNOVATIVE PROPERTIES CO
Al2O3-Y2O3-ZrO2/HfO2 materials, and methods of making and using the same
InactiveUS20030110708A1Facilitates formation and homogeneityOxide formationPigmenting treatmentGlass drawing apparatusFiberThermal insulation
Al2O3-Y2O3-ZrO2 / HfO2 ceramics (including glasses, crystalline ceramics, and glass-ceramics) and methods of making the same. Ceramics according to the present invention can be made, formed as, or converted into glass beads, articles (e.g., plates), fibers, particles, and thin coatings. The particles and fibers are useful, for example, as thermal insulation, filler, or reinforcing material in composites (e.g., ceramic, metal, or polymeric matrix composites). The thin coatings can be useful, for example, as protective coatings in applications involving wear, as well as for thermal management. Certain ceramic particles according to the present invention can be are particularly useful as abrasive particles.
Owner:3M INNOVATIVE PROPERTIES CO
Al2O3-rare earth oxide-ZrO2/HfO2 materials, and methods of making and using the same
InactiveUS20030126803A1Facilitates formation and homogeneityOxide formationPigmenting treatmentOther chemical processesFiberThermal insulation
Al2O3-rare earth oxide-ZrO2 / HfO2 ceramics (including glasses, crystalline ceramics, and glass-ceramics) and methods of making the same. Ceramics according to the present invention can be made, formed as, or converted into glass beads, articles (e.g., plates), fibers, particles, and thin coatings. The particles and fibers are useful, for example, as thermal insulation, filler, or reinforcing material in composites (e.g., ceramic, metal, or polymeric matrix composites). The thin coatings can be useful, for example, as protective coatings in applications involving wear, as well as for thermal management. Certain ceramic particles according to the present invention can be are particularly useful as abrasive particles.
Owner:3M INNOVATIVE PROPERTIES CO
Abrasive particles and methods of making and using the same
InactiveUS20030110706A1Facilitates formation and homogeneityOxide formationPigmenting treatmentOther chemical processesOxideMetal
Abrasive particles comprising ceramic (including glasses, crystalline ceramics, and glass-ceramics) comprising (on a theoretical oxide basis) Al2O3 and at least one other metal oxide (e.g., REO and; REO and at least one of ZrO2 or HfO2) and methods of making the same. The abrasive particles can be incorporated into a variety of abrasive articles, including bonded abrasives, coated abrasives, nonwoven abrasives, and abrasive brushes.
Owner:3M INNOVATIVE PROPERTIES CO
Magnesium based amorphous alloy having improved glass forming ability and ductility
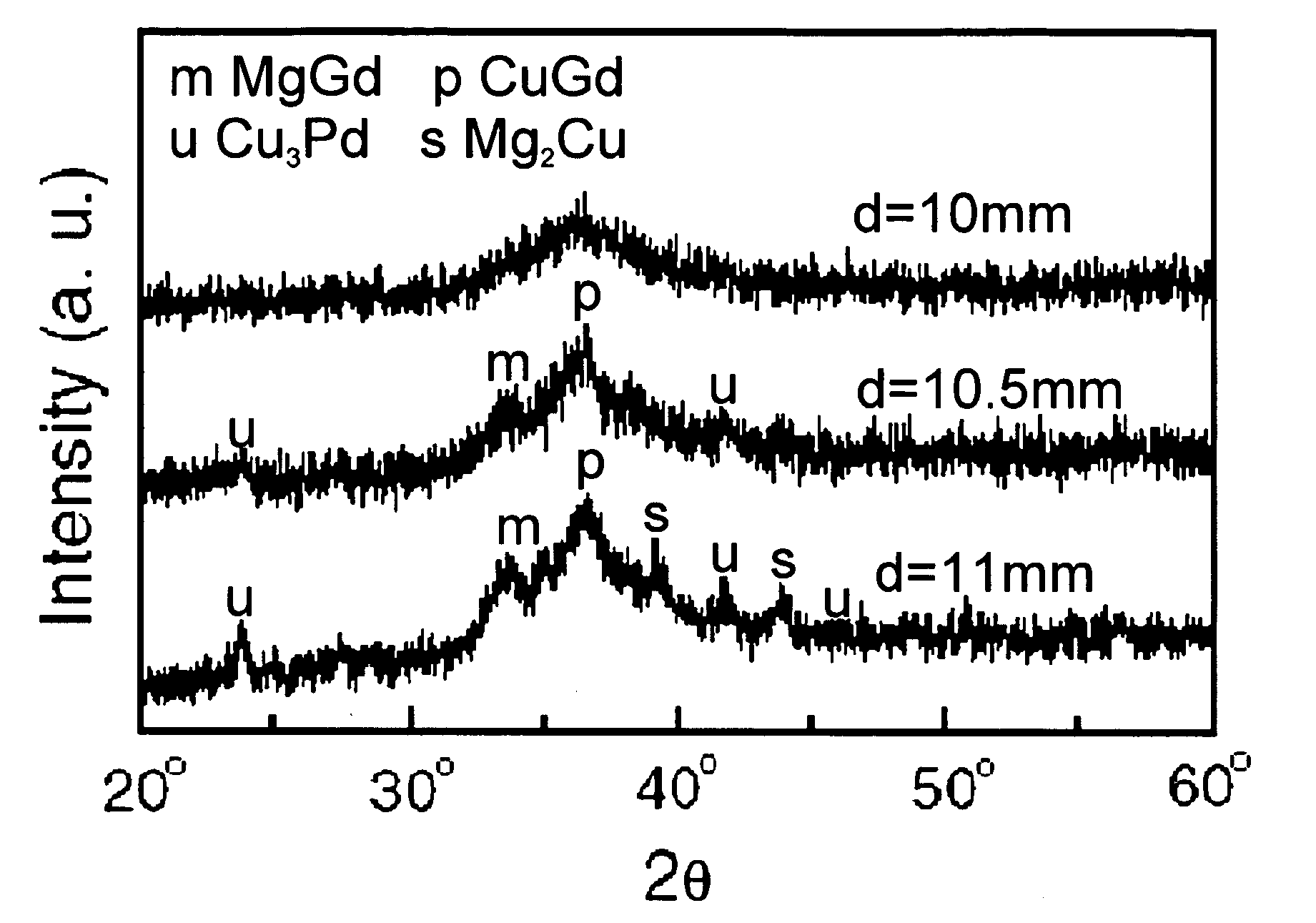
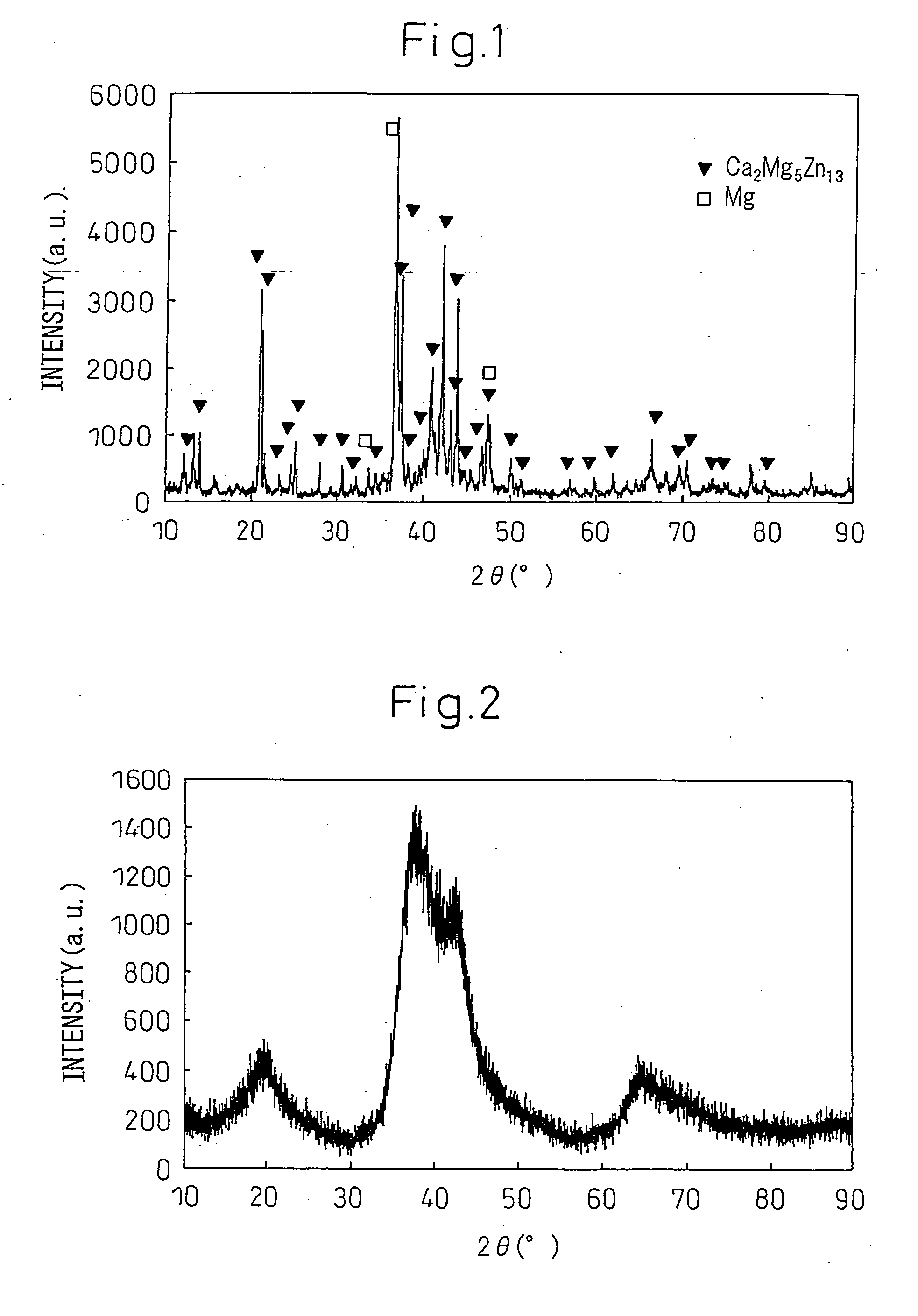
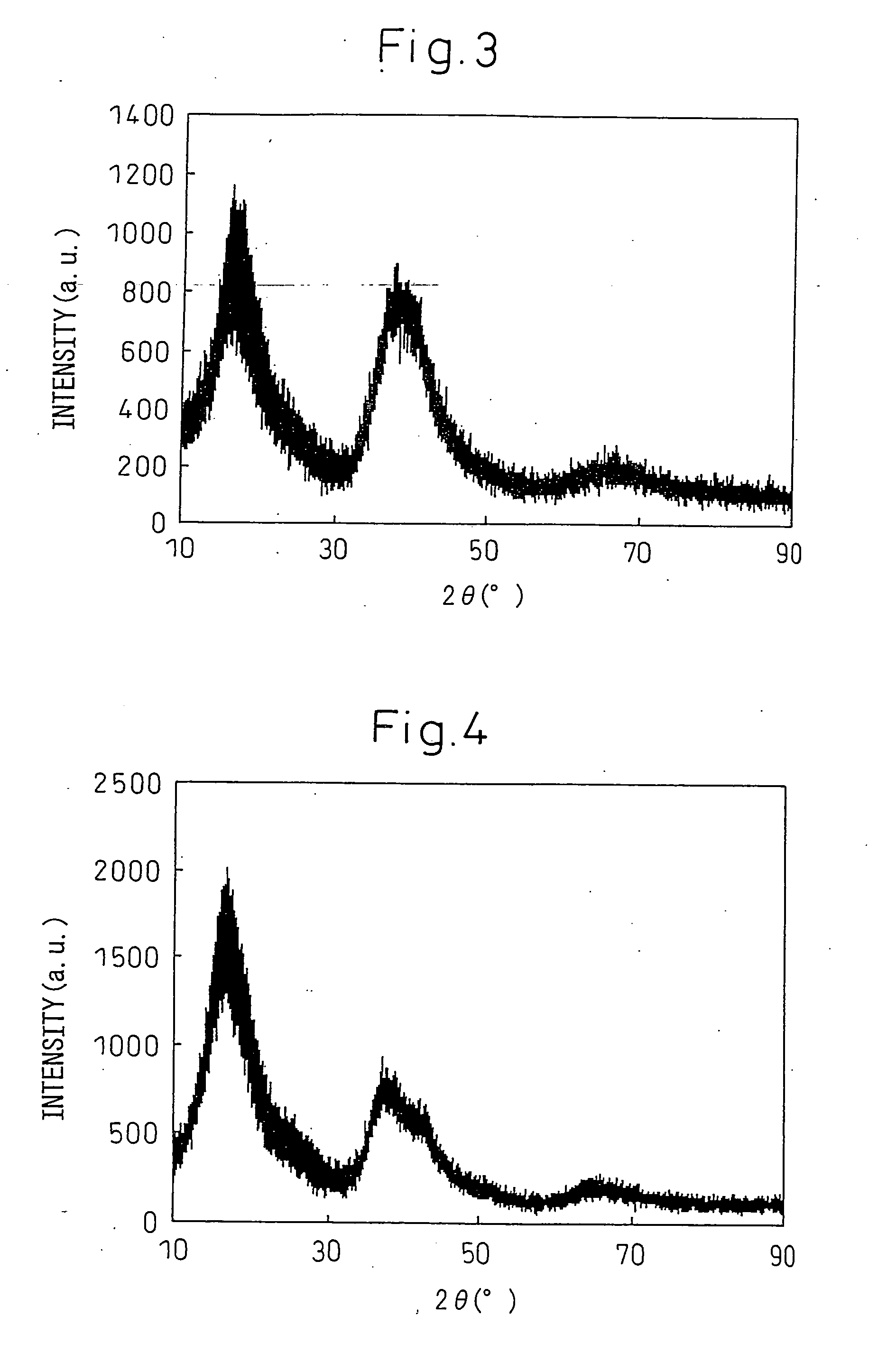
Disclosed is a magnesium based amorphous alloy having a good glass forming ability and ductility. The Mg based amorphous alloy has a composition range of Mg100-x-yAxBy where x and y are respectively 2.5≦x≦30, 2.5≦y≦20 in atomic percent. Here, A includes at least one element selected from the group consisting of Cu, Ni, Zn, Al, Ag, and Pd, and B includes at least one element selected from the group consisting of Gd, Y, Ca, and Nd.
Owner:SAMSUNG ELECTRONICS CO LTD
Systems and methods for implementing bulk metallic glass-based macroscale compliant mechanisms
ActiveUS20140020794A1High glass forming abilityImprove toughnessMetal working apparatusMetalCompliant mechanism
Systems and methods in accordance with embodiments of the invention implement bulk metallic glass-based macroscale compliant mechanisms. In one embodiment, a bulk metallic glass-based macroscale compliant mechanism includes: a flexible member that is strained during the normal operation of the compliant mechanism; where the flexible member has a thickness of 0.5 mm; where the flexible member comprises a bulk metallic glass-based material; and where the bulk metallic glass-based material can survive a fatigue test that includes 1000 cycles under a bending loading mode at an applied stress to ultimate strength ratio of 0.25.
Owner:CALIFORNIA INST OF TECH
Copper-zirconium based amorphous alloy, and preparation method
InactiveCN1958831AImprove mechanical propertiesHigh plastic deformationMolten metal pouring equipmentsElectric arc furnaceAmorphous metal
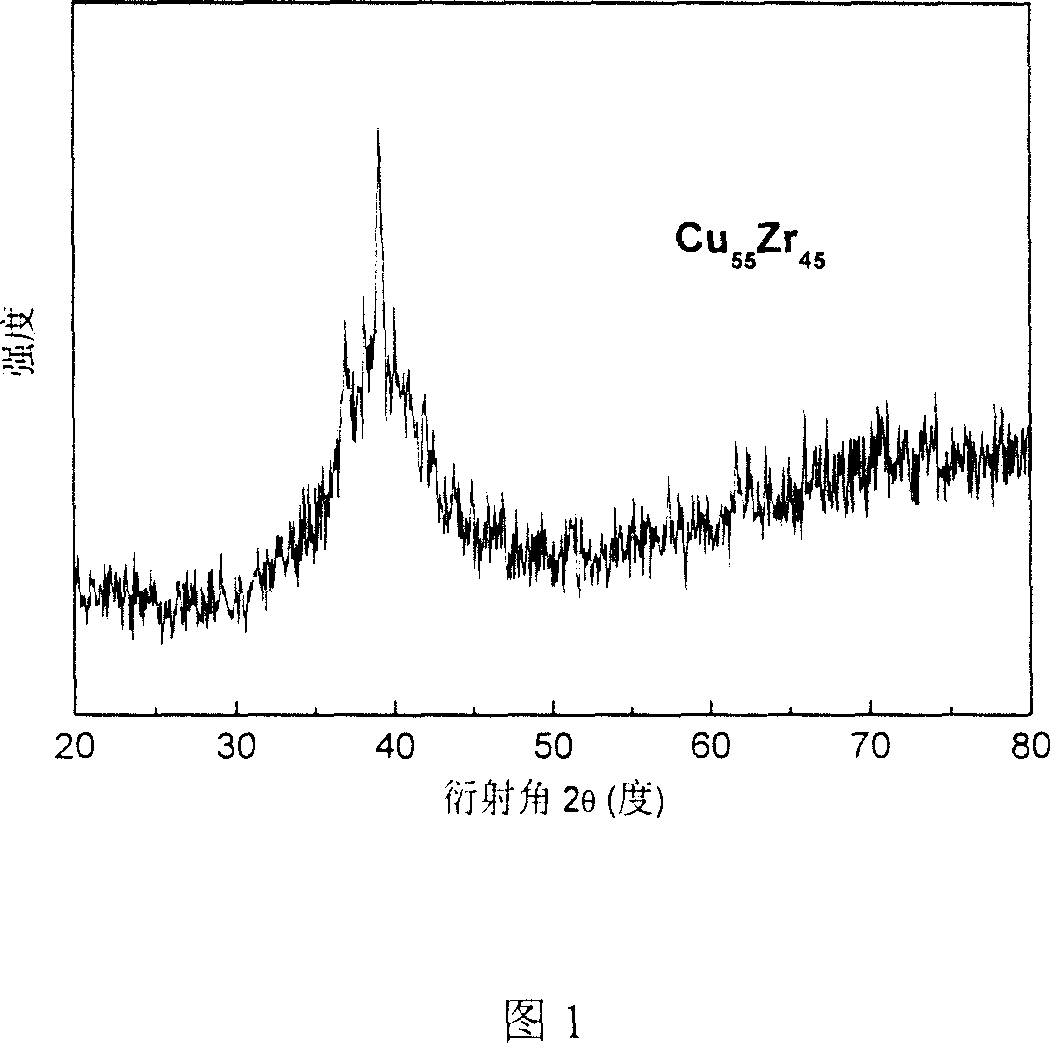
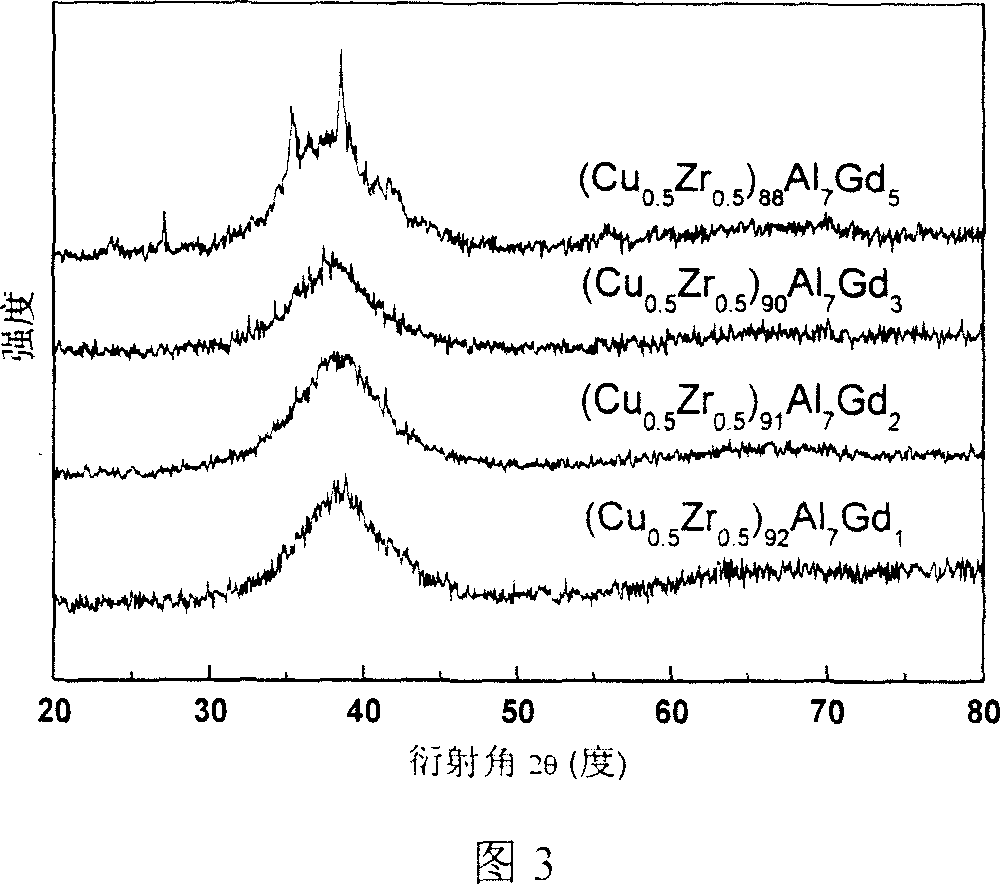
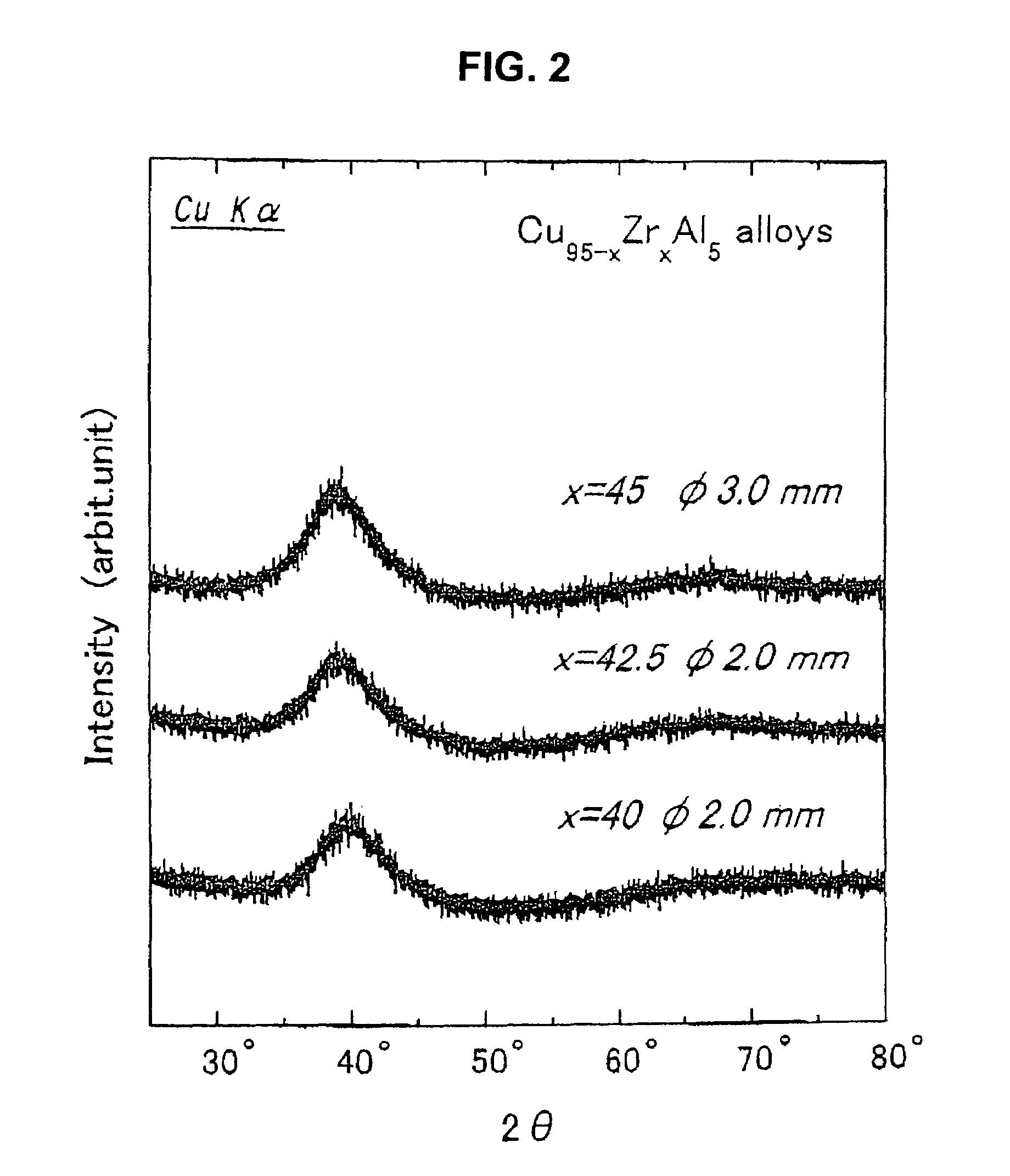
This invention relates to a Cu-Zr-based amorphous alloy, whose general formula is (Cu1-xZrx)aAlbMc, where, x is 0.40-0.60; a is 80-100; b is 0-14; c is 0-20; a + b + c is 100; M is Y, La, Ce, Pr, Nd, Gd, Tb, Dy, Ho, Er, Ti, Ag, Ga, Hf, Ta, Nb, Ni, Co or Fe. The preparation method comprises: (1) mixing Cu, Zr, Al and M according to the ratio in Ti-adsorbed Ar atmosphere in an arc furnace, smelting and cooling to obtain mother alloy ingot; (2) re-melting the mother alloy ingot in air, and casting into a water-cooling metal mold to obtain the Cu-Zr-based amorphous alloy. The alloy has high glass formation ability, high crystallization inhibition and large size at a very low cooling speed.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Amorphous metal deposition and new aluminum-based amorphous metals
InactiveUS20050123686A1Improve toughnessIncrease impactLiquid surface applicatorsMolten spray coatingHigh densityMetal alloy
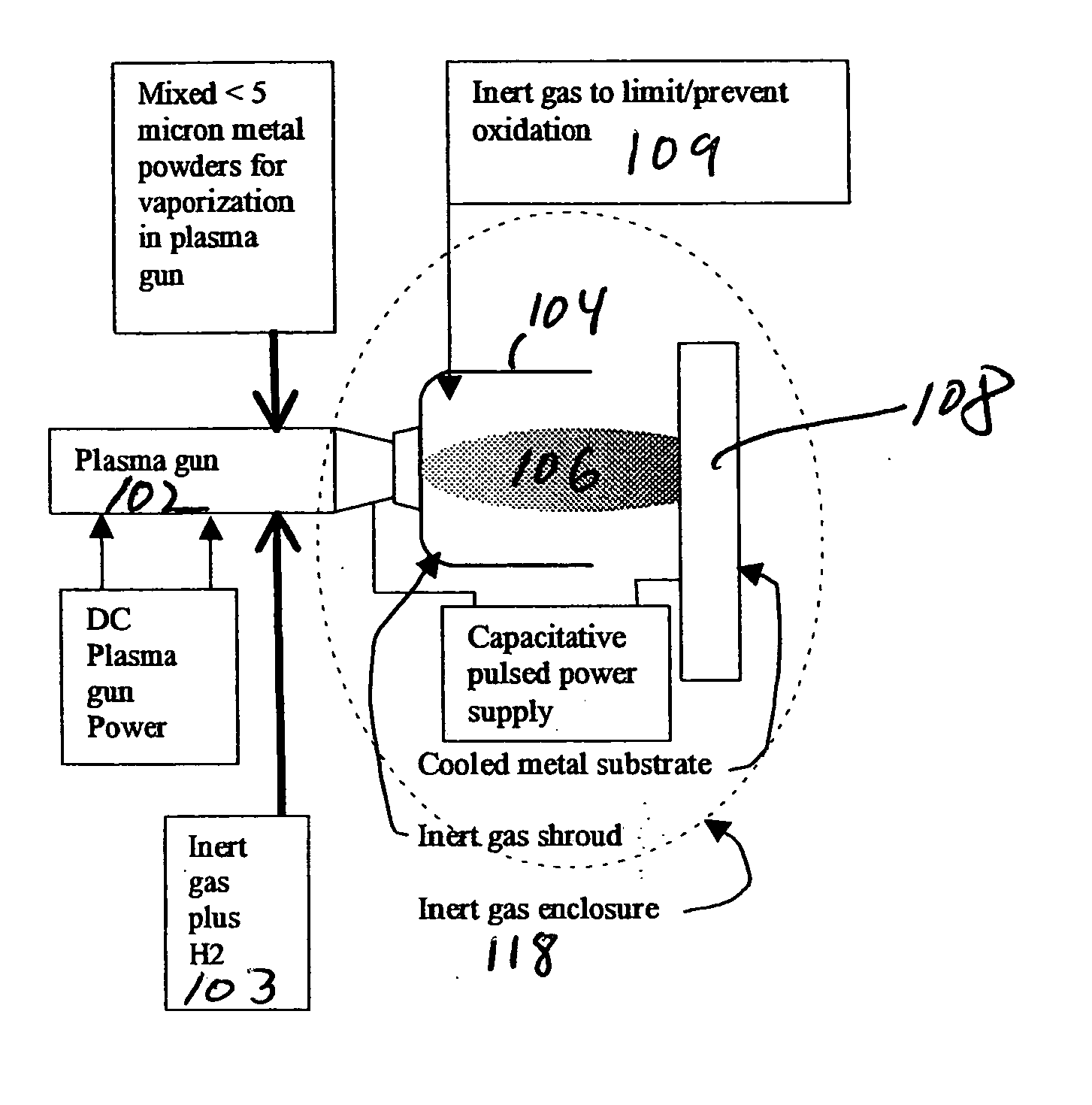
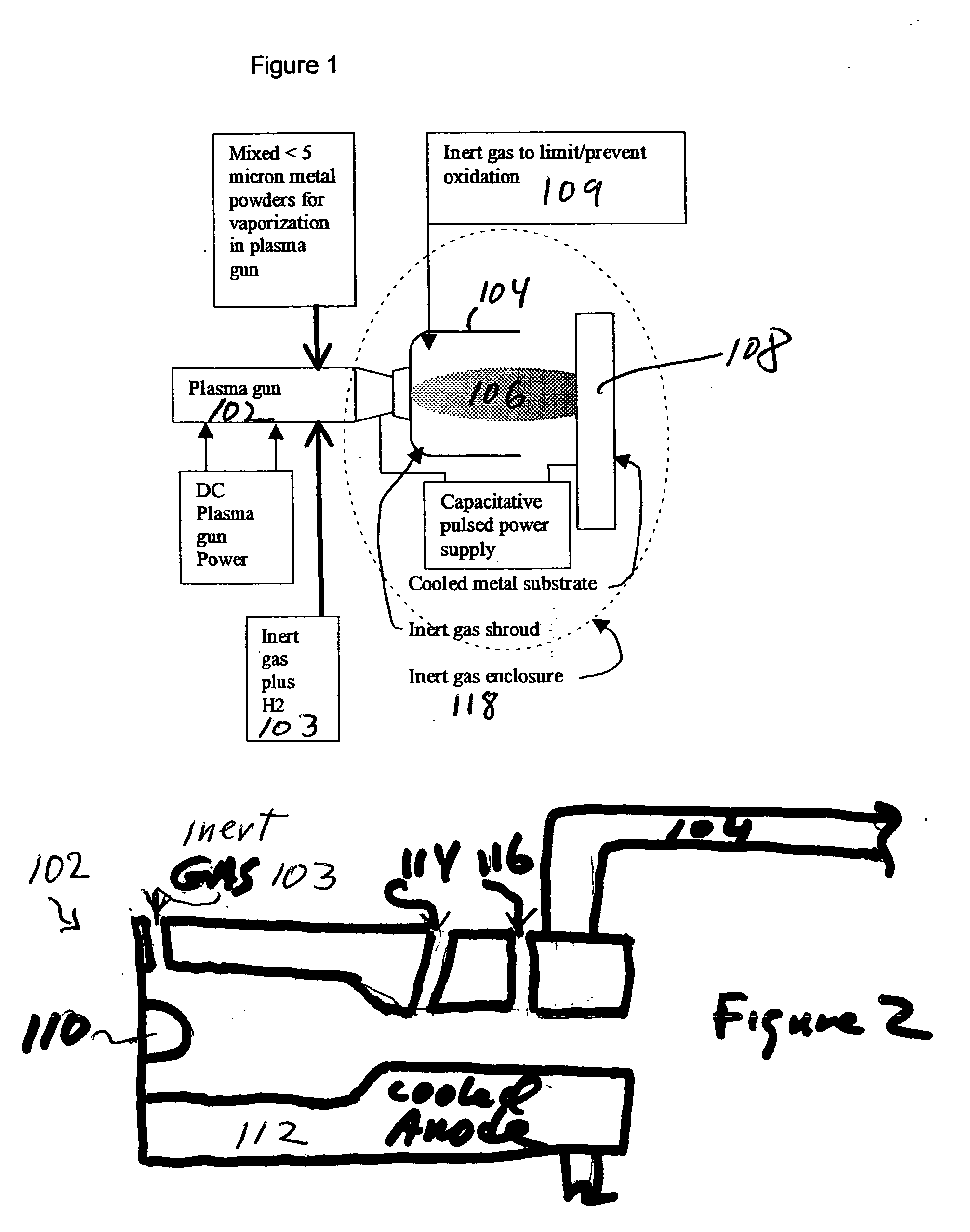
Methods for applying an amorphous metal alloy to a substrate, comprising the steps of vaporizing an amorphous metal alloy composition, in a plasma spray gun to form a metal alloy vapor plasma plume, directing the metal alloy vapor plume onto a cooled substrate, maintained and condensing and rapidly solidifying the amorphous metal alloy composition vapor on the substrate, to form an amorphous metal layer deposit of high density and strength.
Owner:MYRICK JAMES J
Active amorphous brazing filler metal for brazing ZrB2-SiC ceramic materials, preparation method for active amorphous brazing filler metal and brazing process
InactiveCN105252169AImprove wettabilityImprove toughnessWelding/cutting media/materialsSoldering mediaUltra-high-temperature ceramicsRoom temperature
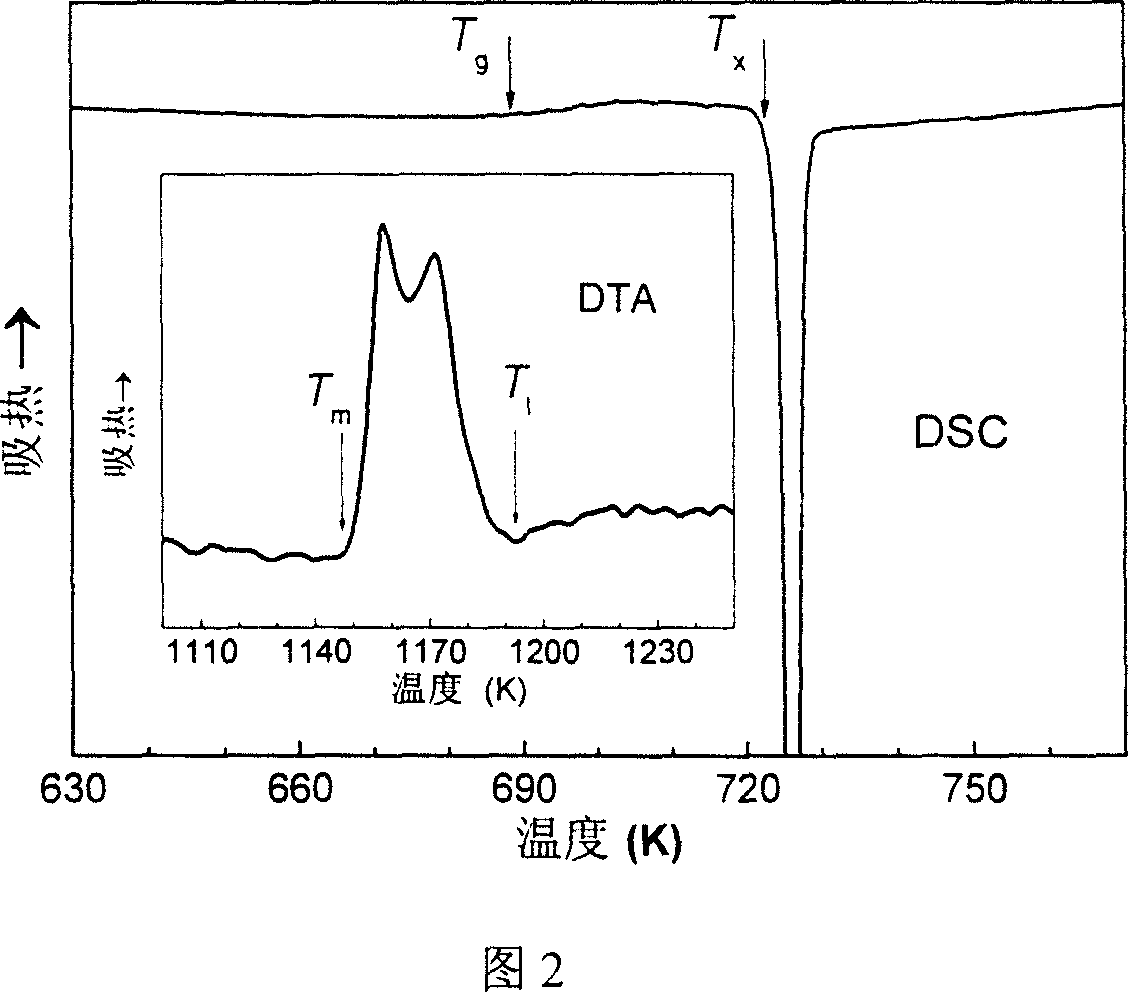
The invention discloses active amorphous brazing filler metal for brazing ZrB2-SiC ceramic materials, a preparation method for the active amorphous brazing filler metal and a brazing process, particularly relates to Cu-Ti-Ni-Zr high-temperature active amorphous brazing filler metal for brazing ZrB2-SiC ultra-high-temperature ceramic materials, a preparation method for the Cu-Ti-Ni-Zr high-temperature active amorphous brazing filler metal and a brazing process, and belongs to brazing filler metal in the amorphous and metallurgy fields. The components of the brazing filler metal comprise, by atomic percentage, 36.0-42.0% of Cu, 30.0-35.0% of Ti, 16.0-23.0% of Zr and the balance Ni. The melting temperature of the active amorphous brazing filler metal is 1110-1150 K, and the brazing temperature is 1183-1273 K. The Cu-Ti-Ni-Zr high-temperature active amorphous brazing filler metal obtained through the rapid condensation technology has good wettability. The room temperature shearing strength of the ZrB2-SiC ultra-high-temperature ceramic materials brazed through the amorphous brazing filler metal in a vacuum brazing mode can be as high as 160 MPa and much higher than that of Cu-based and Ag-based brazing filler metal.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Systems and methods for implementing bulk metallic glass-based macroscale compliant mechanisms
ActiveUS9783877B2High glass forming abilityImprove toughnessMetal working apparatusCompliant mechanismUltimate tensile strength
Systems and methods in accordance with embodiments of the invention implement bulk metallic glass-based macroscale compliant mechanisms. In one embodiment, a bulk metallic glass-based macroscale compliant mechanism includes: a flexible member that is strained during the normal operation of the compliant mechanism; where the flexible member has a thickness of 0.5 mm; where the flexible member comprises a bulk metallic glass-based material; and where the bulk metallic glass-based material can survive a fatigue test that includes 1000 cycles under a bending loading mode at an applied stress to ultimate strength ratio of 0.25.
Owner:CALIFORNIA INST OF TECH
Amorphous nickel-free zirconium alloy
An amorphous Nickel-Free Zirconium alloy which is readily formed through copper mold casting, comprising a composition consisting of four elements in which the first element is Zr, the second element is Ti, the third element is Cu and the fourth element is Al, wherein an atomic percent of the first to the fourth elements in the composition are represented by a, b, c and d respectively, wherein a=45˜69%, b=0.25˜8%, c=21˜35%, and d=7.5˜15%, where a sum of a, b, c and d is smaller than or equal to 100%. The composition of the amorphous alloy within the above range is melted in a copper mold to form bulk amorphous materials or parts which have characteristics of high tensile strength, high fracture toughness, low Young's modulus and high corrosion resistance.
Owner:INST OF METAL RES CHINESE ACADEMY OF SCIENECE
Fe-Based Bulk Amorphous Alloy Compositions Containing More Than 5 Elements And Composites Containing The Amorphous Phase
Disclosed is a Fe-based bulk amorphous alloy composition which forms a bulk amorphous substance due to its excellent amorphous formability when it is cooled to a temperature lower than its glass transition temperature from the liquid state at a relatively low cooling rate of 1000 K / s or less, has high warm processability in a low temperature range owing to its supercooled liquid region of 20K or higher and has excellent fluidity in the liquid state and thereby good castability. The Fe-based multi-element bulk amorphous alloy composition is represented by a formula of Feα, CβSiγBxPyMa, in which M is at least one element selected from Ti (titanium), Cr (chromium), Mo (molybdenum), Nb (niobium), Zr (Zirconium), Ta (tantalum), W (tungsten) and V (vanadium), α, β, γ, x, y, and a each represent atomic % of iron (Fe), Carbon (C), silicon (Si), boron (B), phosphorus (P) and the selected metal element, in which α is 100-(β+γ+x+y+a) atomic %, β is 6 atomic % or more and 13 atomic % or less, γ is 1 atomic % or more and 5 atomic % or less, x is 4.5 atomic % or more and 9.5 atomic % or less, y is 3 atomic % or more and 10 atomic % or less and a is 0.1 atomic % or more and 6 atomic % or less.
Owner:POHANG IRON & STEEL CO LTD
Bulk nickel-silicon-boron glasses bearing iron
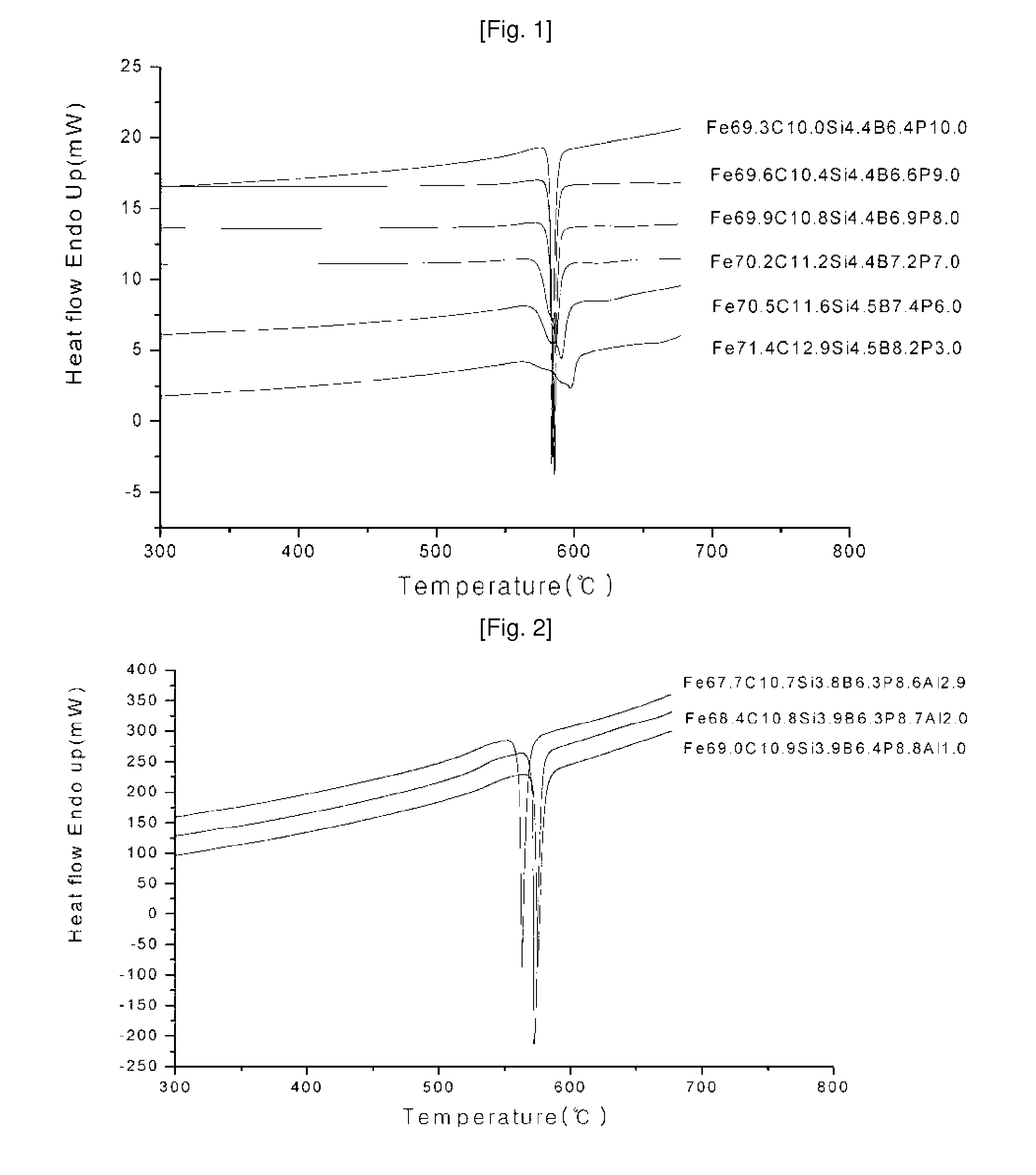
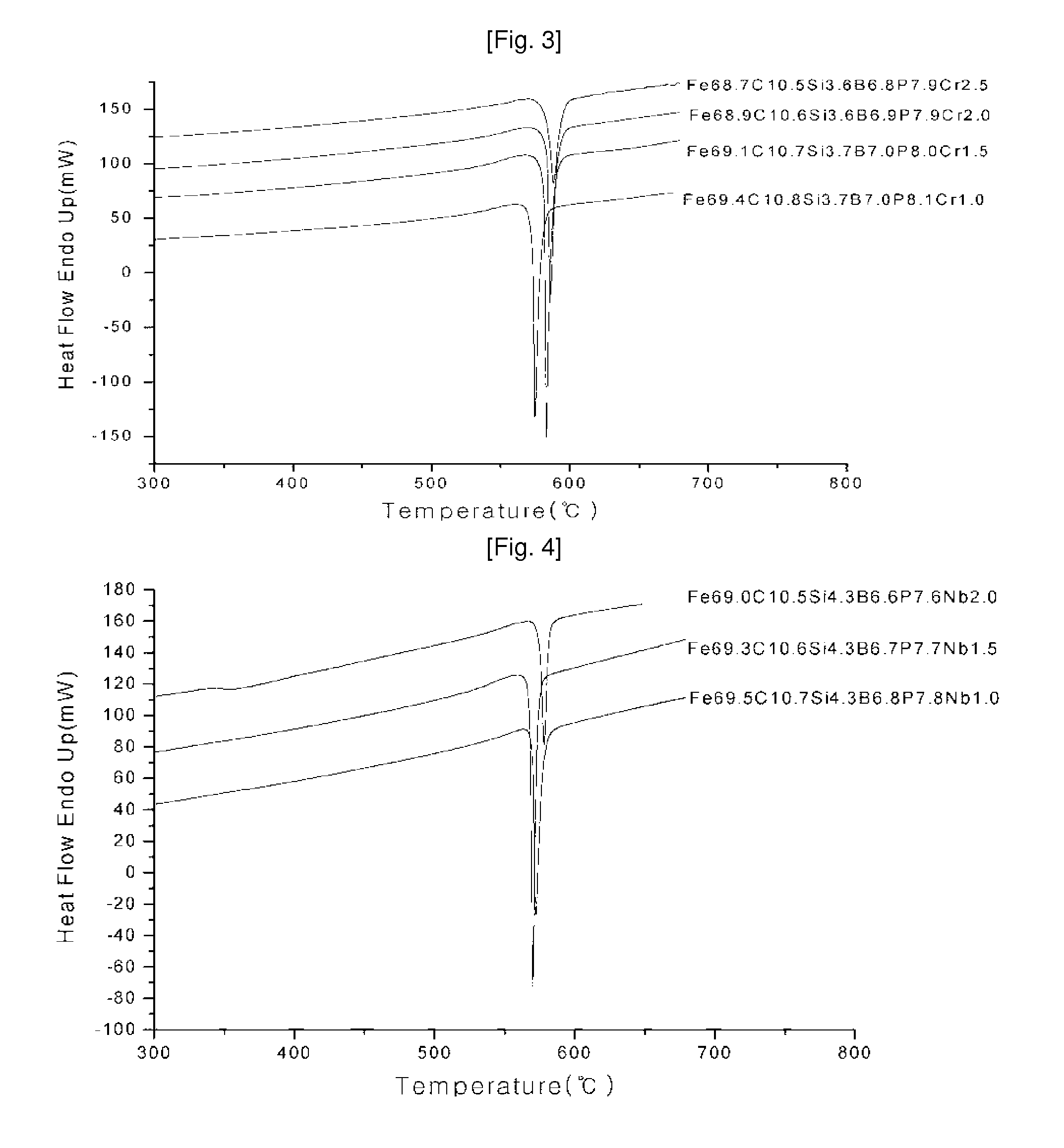
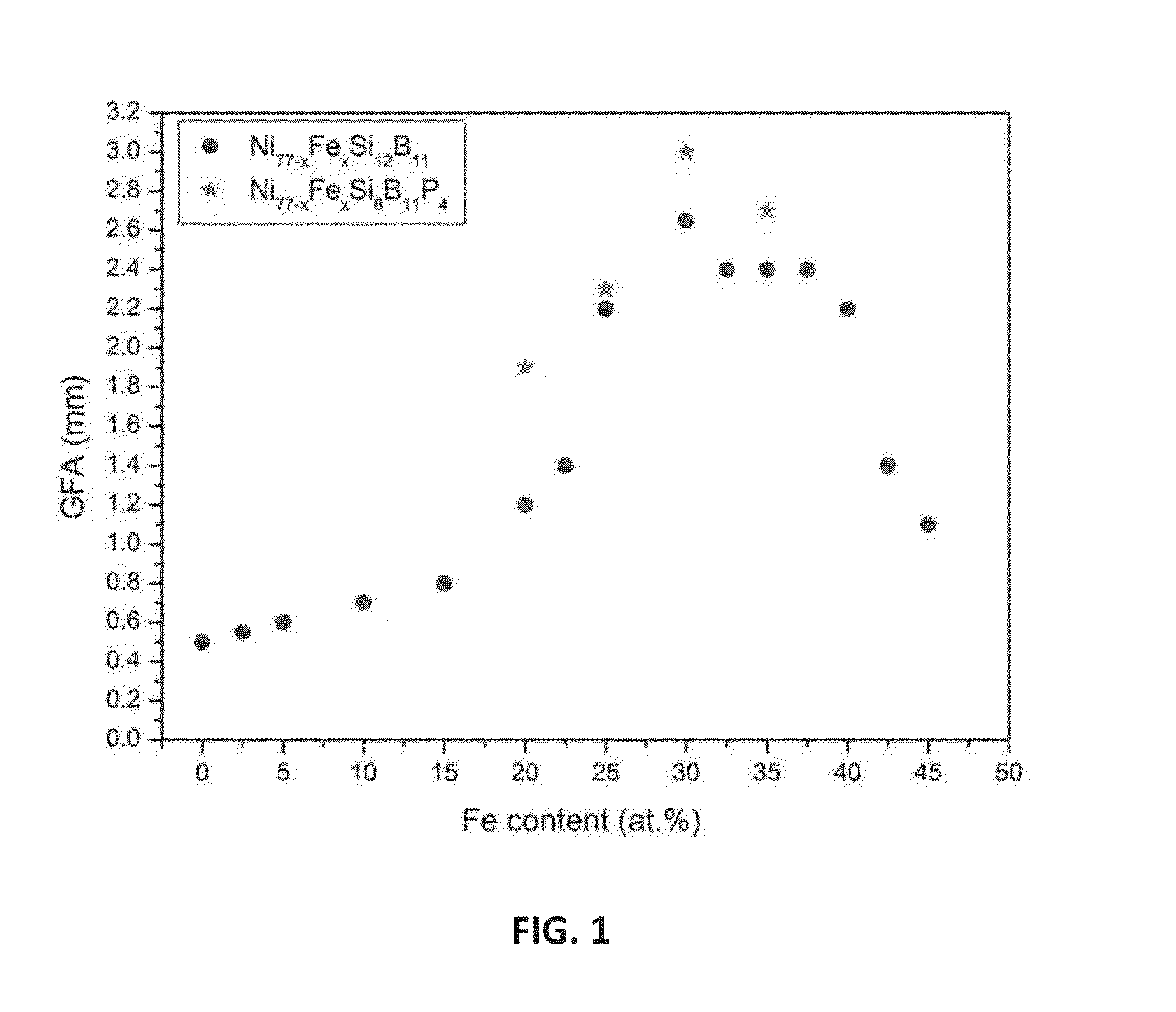
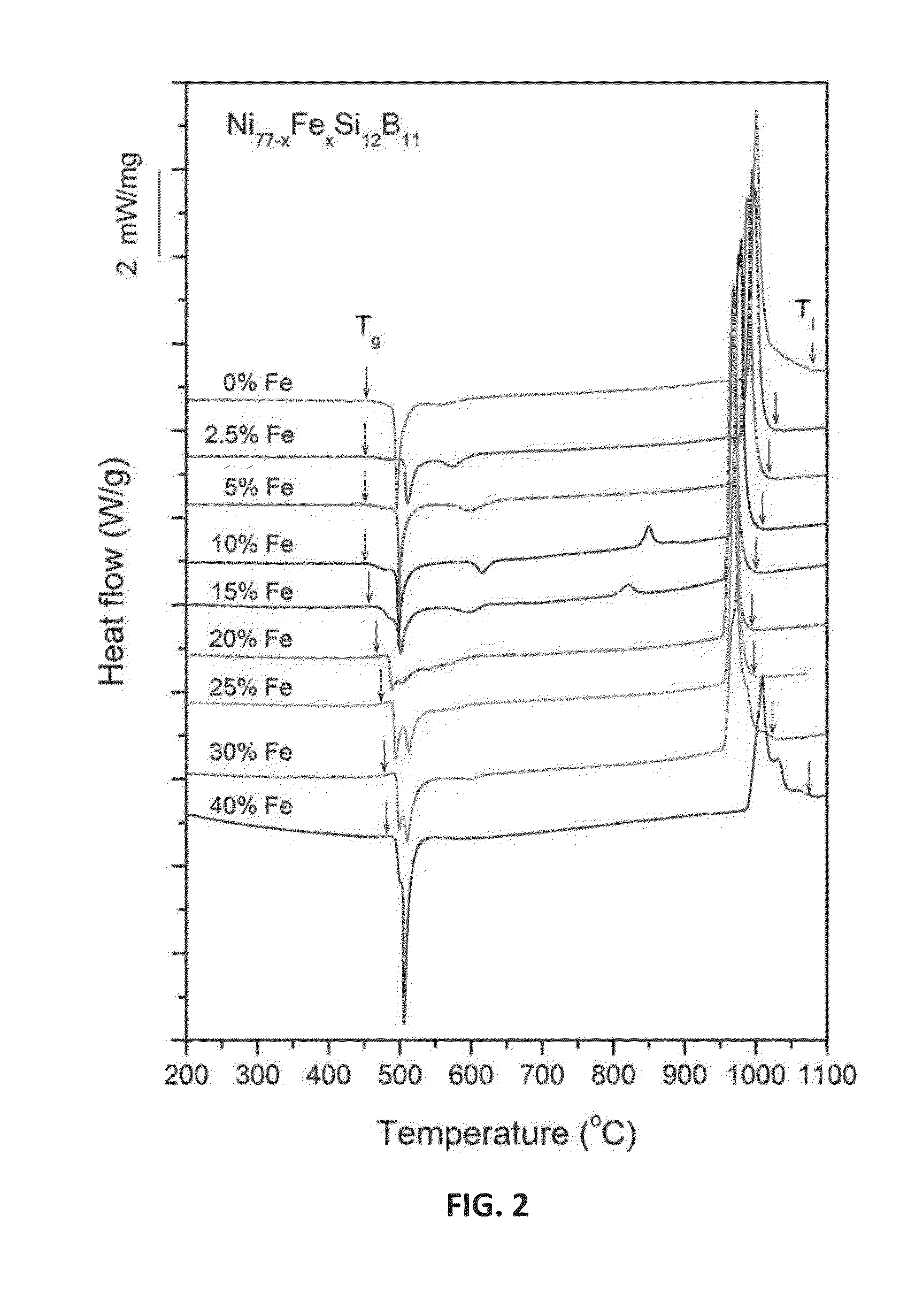
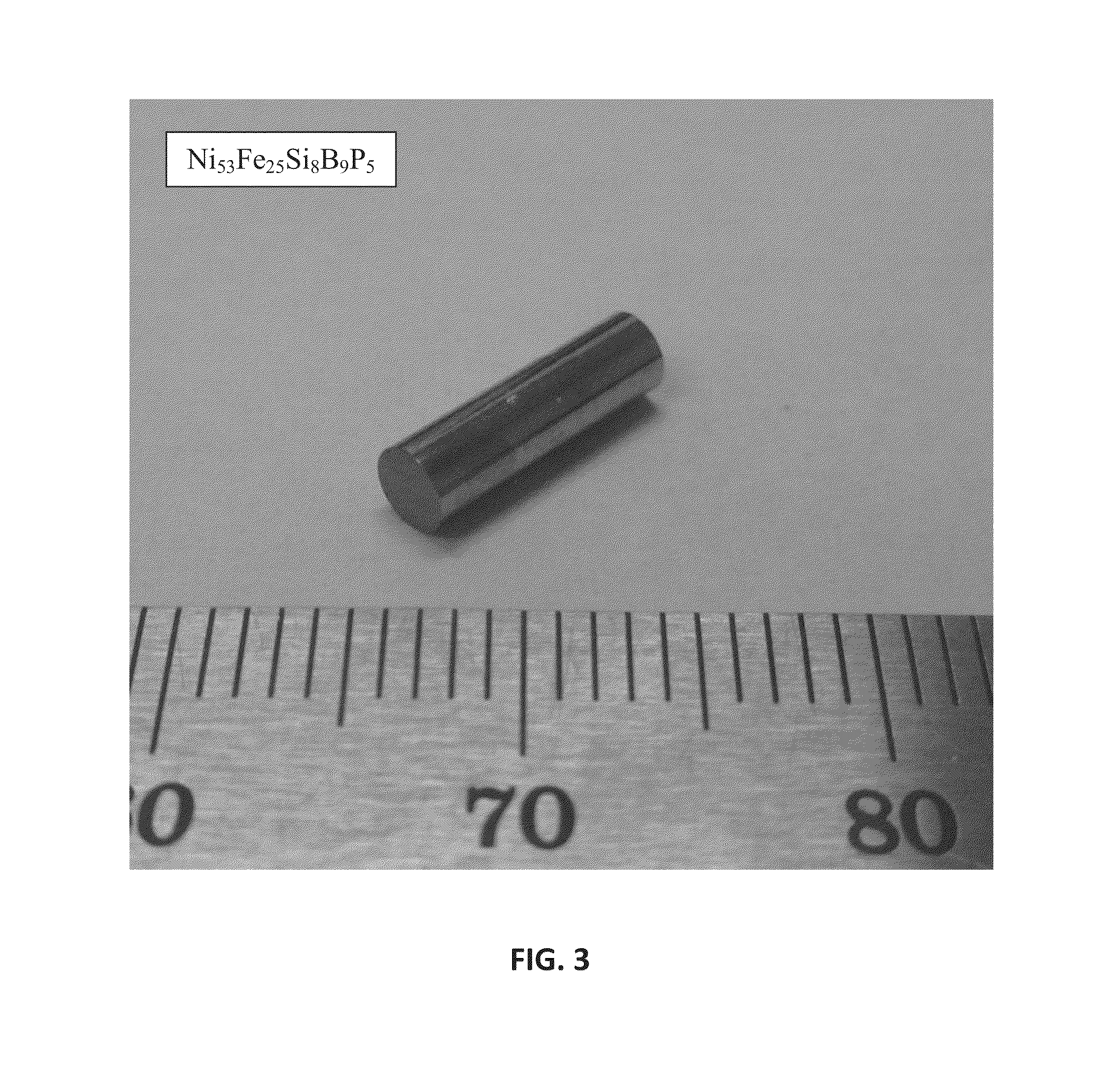
Ni—Fe—Si—B and Ni—Fe—Si—B—P metallic glass forming alloys and metallic glasses are provided. Metallic glass rods with diameters of at least one, up to three millimeters, or more can be formed from the disclosed alloys. The disclosed metallic glasses demonstrate high yield strength combined with high corrosion resistance, while for a relatively high Fe contents the metallic glasses are ferromagnetic.
Owner:APPLE INC
Cerium-base bulk amorphous alloys and method for preparation thereof
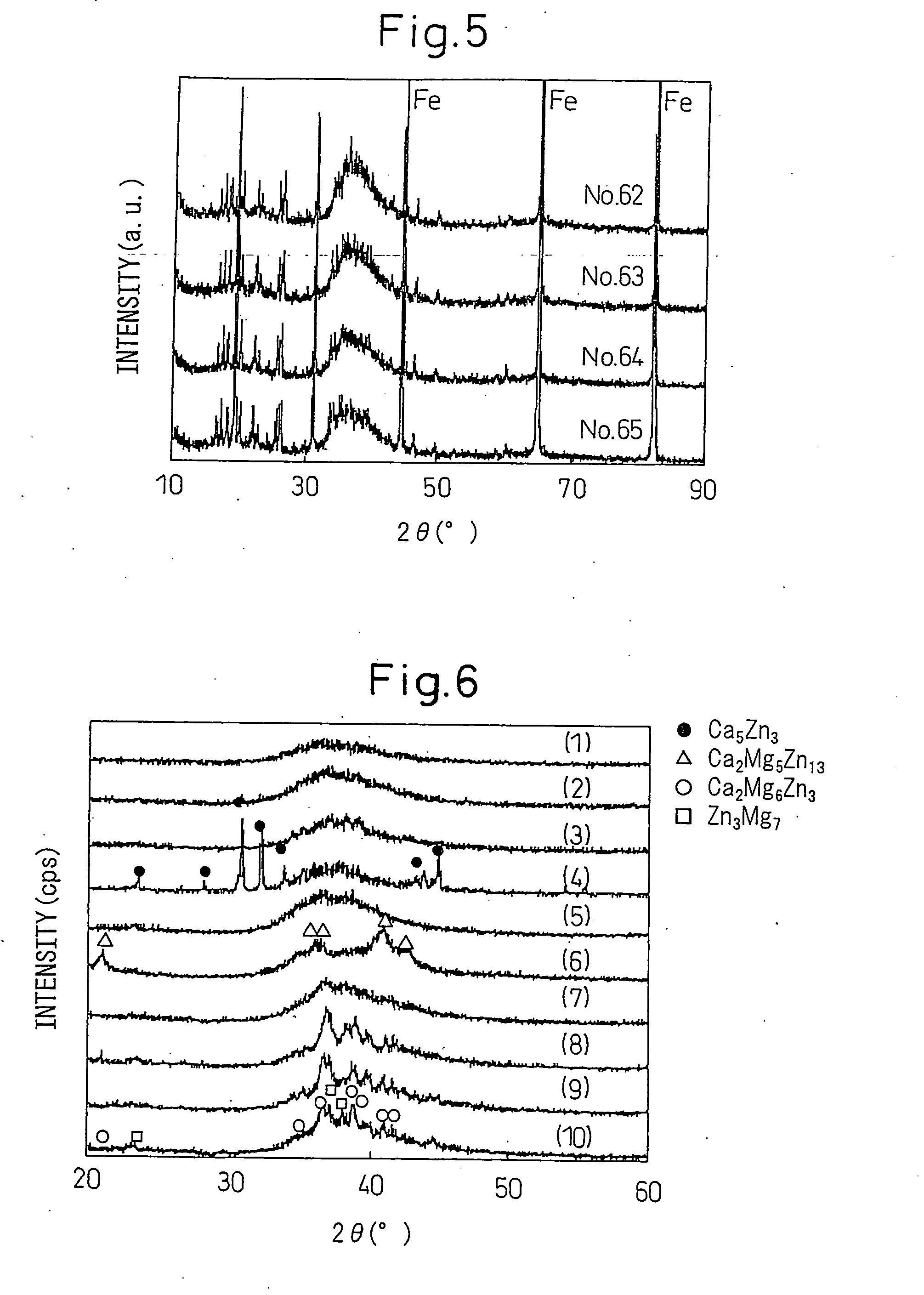
The invention relates to a sort of cerium-based block amorphous alloy, which is formed primarily from the cerium and contains at least 50% amorphous phase percentage by volume, and can be represented by the formula: CeaAlbNicCudMe, wherein 50<=a<=80, 5<=b<=20, 5<=c<=20, 5<=d<=20, 0<=e<=10, and a+b+c+d+e=100, and said transition metal elements M is Sc, Nb, Ti, V, Cr, Mn, Fe, Co, Y, Zr, Mo, La, Pr , Nd or Hf. The preparation of the alloy is in the electric arc furnace of argon atmosphere absorbed with the titanium and the steps is: smelting each group according to needed atomic matching, mixing them evenly, and getting the key metal ingoting after cooling, smelting the ingoting again by normal chill casting method, inhaling the flux to the water-cooled copper mould through the suction apparatus in the furnace, then getting the alloy.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Preparation method of Sm-Co-based amorphous nanocrystalline thin-strip magnet
ActiveCN102403117AHigh glass forming abilityHigh curie temperaturePermanent magnetsInductances/transformers/magnets manufactureMagnetic transitionsHigh resistance
The invention relates to a preparation method of a Sm-Co-based amorphous nanocrystalline thin-strip magnet, and relates to a rare earth metal and magnetic transition metal containing magnet made of a hard magnetic material. The Sm-Co-based amorphous nanocrystalline thin-strip magnet has a chemical formula of SmxCoyFezZruBvQw, wherein the symbols of qualified elements metered by atomic percentage meet the following conditions: x + y + z + u + v + w = 100, x = 9.0-14.0, y = 45.0-70.5, z = 2.8-18.4 , u = 1.1-7.0, v = 3.8-19.0, w = 1.0-21.2, and Q is one to four elements of Nb, Al, Si, Cu and C. The Sm-Co-based amorphous nanocrystalline thin-strip magnet prepared by a centrifugal fast quenching and strip throwing technology has the advantages of high room temperature intrinsic coercivity, high resistance to corrosion and high strength.
Owner:HEBEI UNIV OF TECH
Cu-base amorphous alloy
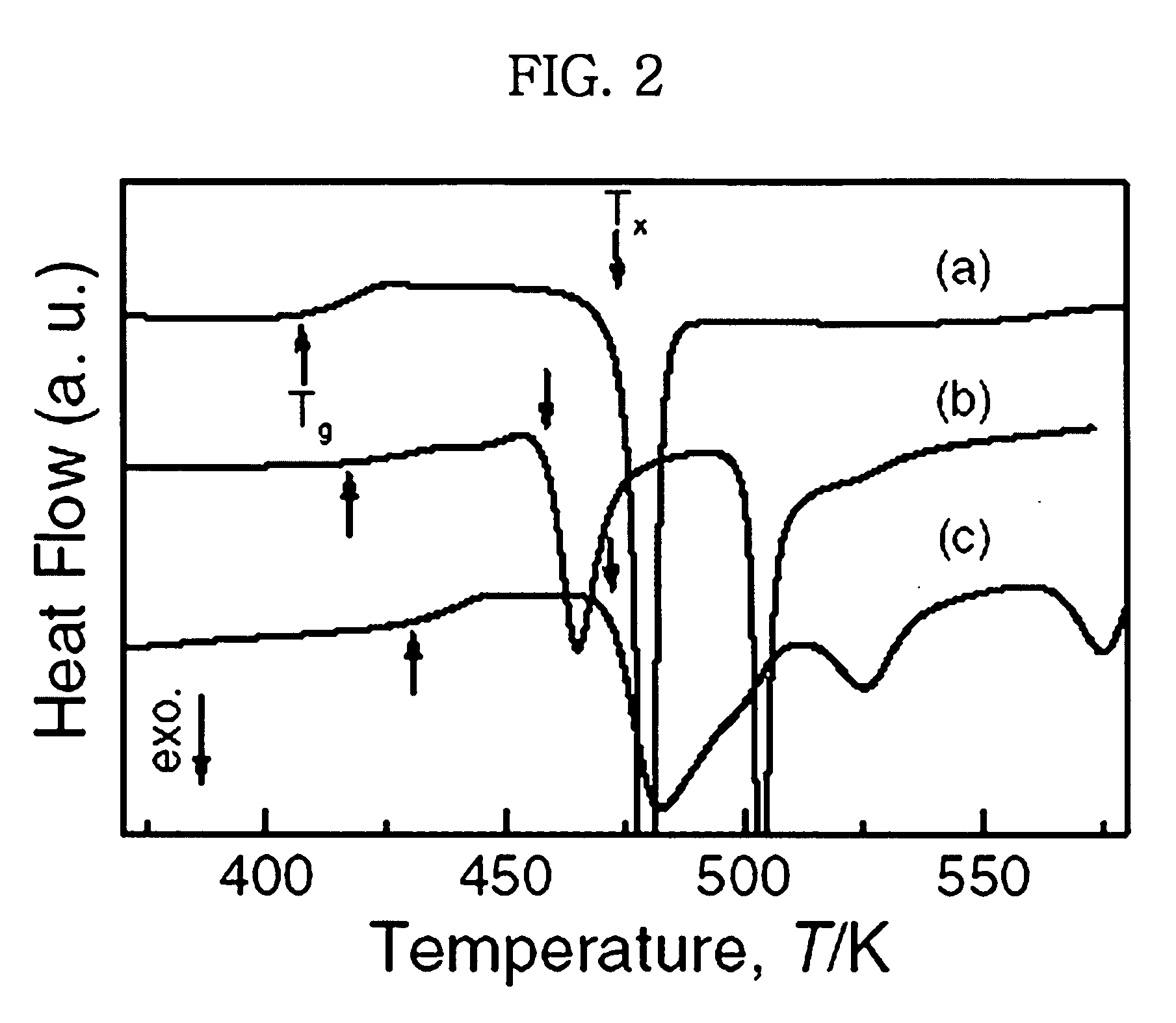
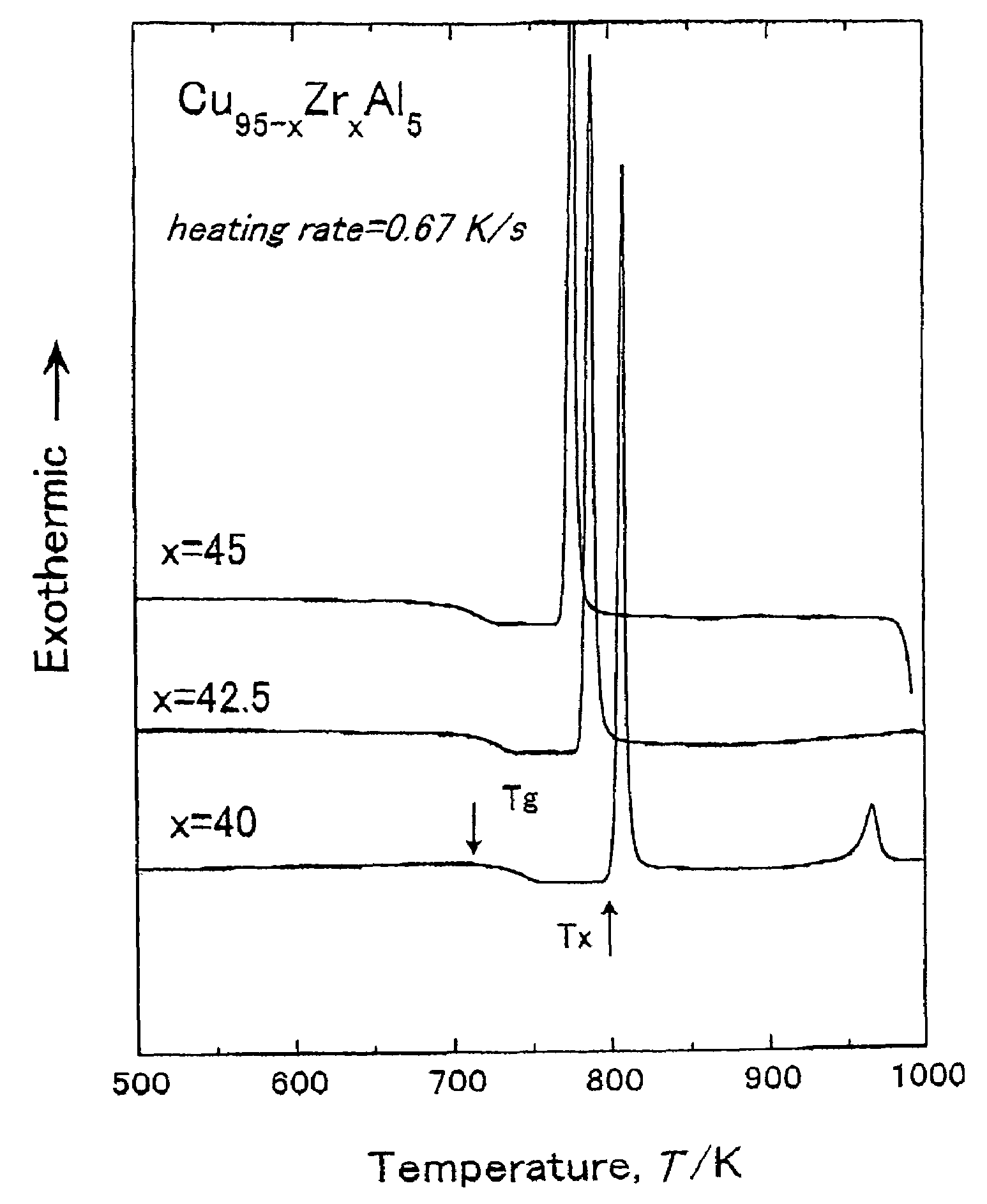
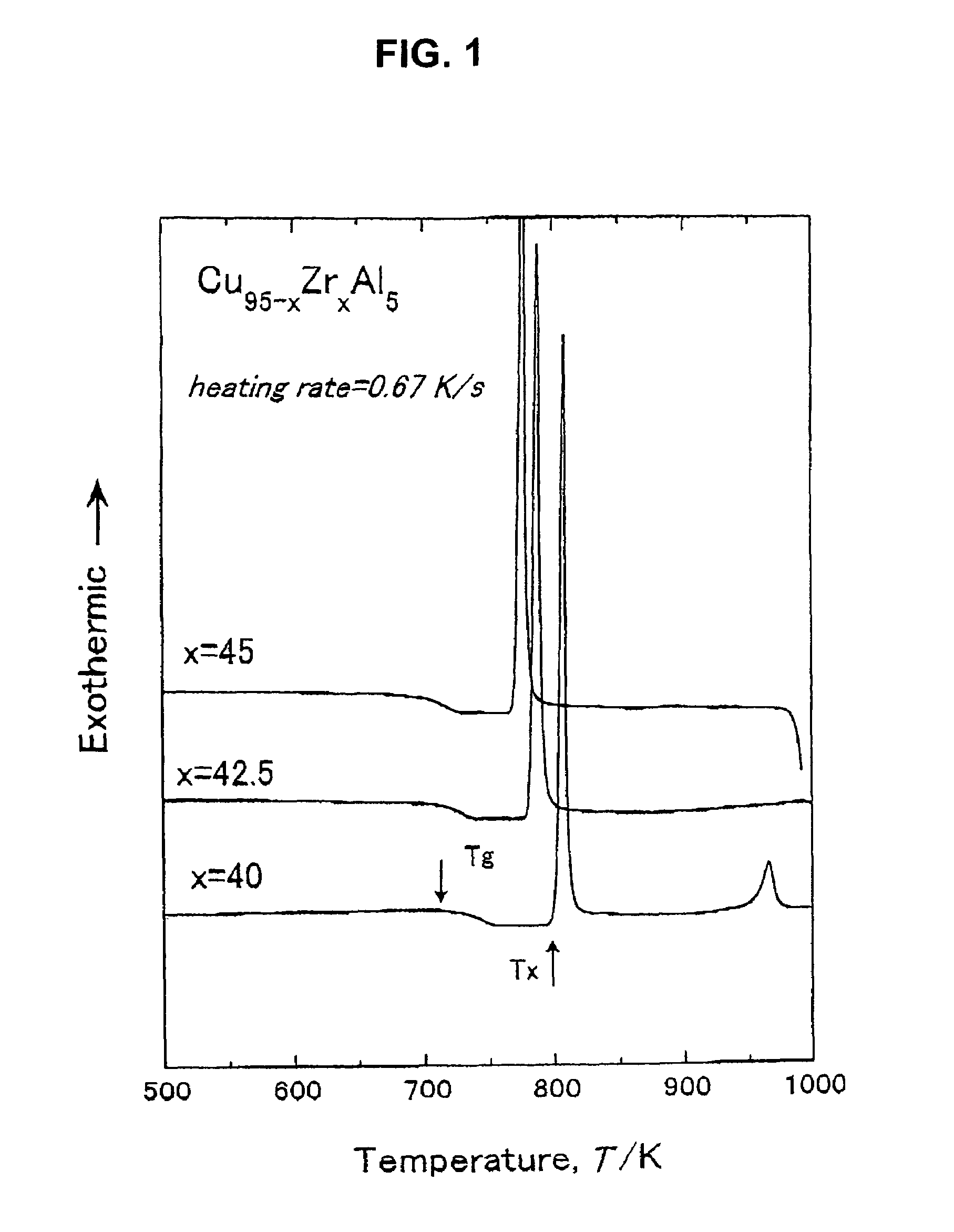
To provide a Cu-based amorphous alloy having a glass-forming ability higher than that of a Cu—Zr—Ti amorphous alloy and a Cu—Hf—Ti amorphous alloy, as well as excellent workability and excellent mechanical properties without containing large amounts of Ti. A Cu-based amorphous alloy characterized by containing 90 percent by volume or more of amorphous phase having a composition represented by Formula: Cu100-a-b(Zr,Hf)a(Al,Ga)b [in Formula, a and b are on an atomic percent basis and satisfy 35 atomic percent≦a≦50 atomic percent and 2 atomic percent≦b≦10 atomic percent], wherein the temperature interval ΔTx of supercooled liquid region is 45 K or more, the temperature interval being represented by Formula ΔTx=Tx−Tg (where Tx represents a crystallization initiation temperature and Tg represents a glass transition temperature.), a rod or a sheet having a diameter or thickness of 1 mm or more and a volume fraction of amorphous phase of 90% or more can be produced by a metal mold casting method, the compressive strength is 1,900 MPa or more, the Young's modulus is 100 GPa or more, and the Vickers hardness is 500 Hv or more.
Owner:JAPAN SCI & TECH CORP
Annealing-induced extensive solid-state amorphization in metallic films
An thin film alloy based on chemical elements with high glass forming ability is disclosed. The alloy is deposited as a thin film from a source of substantially the same chemical composition. Within the deposited thin film, amorphization is induced extensively up to decades of micrometers in size during controlled annealing. Such controllable extensive amorphization throughout the thin film is useful to regulate the proportion of amorphous phase to crystalline phase, establish the structure / property relationships and thus tailor specific properties.
Owner:NATIONAL TAIWAN OCEAN UNIVERSITY
Method for preparing ultrahard erosion-resistant amorphous steel coating
InactiveCN101812657AGood corrosion resistanceGood glass forming abilityMolten spray coatingScrapCorrosion
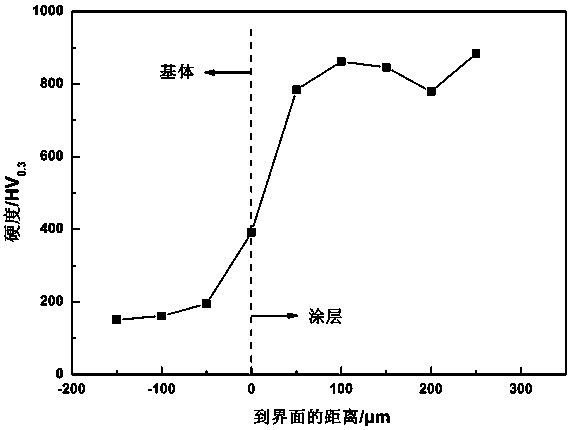
The invention provides a method for preparing ultrahard erosion-resistant amorphous steel coating, which relates to an amorphous steel alloy with good glass-forming capability and the high strength, high hardness and excellent corrosion resistance and erosion resistance of the amorphous steel alloy, in particular to a preparation method for amorphous steel coating and the potential application fields of the amorphous steel coating. The method is characterized in that: amorphous steel powder is prepared by utilizing the ultrasonic gas atomization technique, the amorphous steel coating is prepared by utilizing high-speed three-electrode plasma spraying, the technique is flexible, the cost is low, and industrialization can be easily implemented. The high-performance amorphous steel coating obtained by the method has properties such as compact structure, low oxygen content, extremely high hardness, high corrosion resistance, excellent erosion resistance and the like, and has a great application prospect in fields such as hard chromium substitution, ships, oil and gas fields, hydraulic facilities and nuclear waste treatment. In addition, the possible application range of the technique is wider, and the technique can provide good concept and method for protective costing preparation and has certain guiding significance.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Abrasive particles and methods of making and using the same
InactiveUS7563294B2Facilitates formation and homogeneityEliminates and minimizes heat transferPigmenting treatmentGlass drawing apparatusGlass-ceramicMetal
Abrasive particles comprising ceramic (including glasses, crystalline ceramics, and glass-ceramics) comprising (on a theoretical oxide basis) Al2O3 and at least one other metal oxide (e.g., REO and; REO and at least one of ZrO2 or HfO2) and methods of making the same. The abrasive particles can be incorporated into a variety of abrasive articles, including bonded abrasives, coated abrasives, nonwoven abrasives, and abrasive brushes.
Owner:3M INNOVATIVE PROPERTIES CO
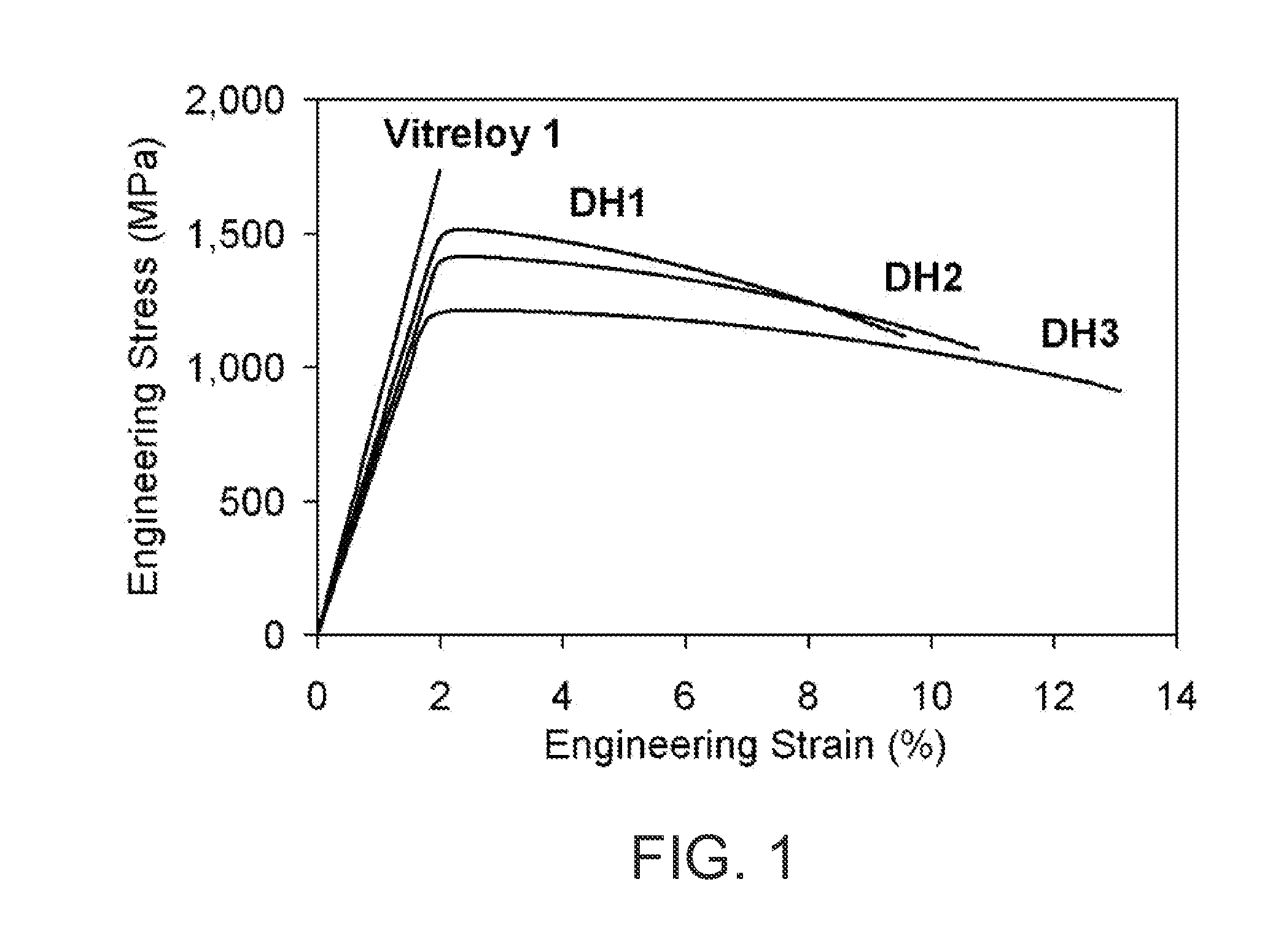
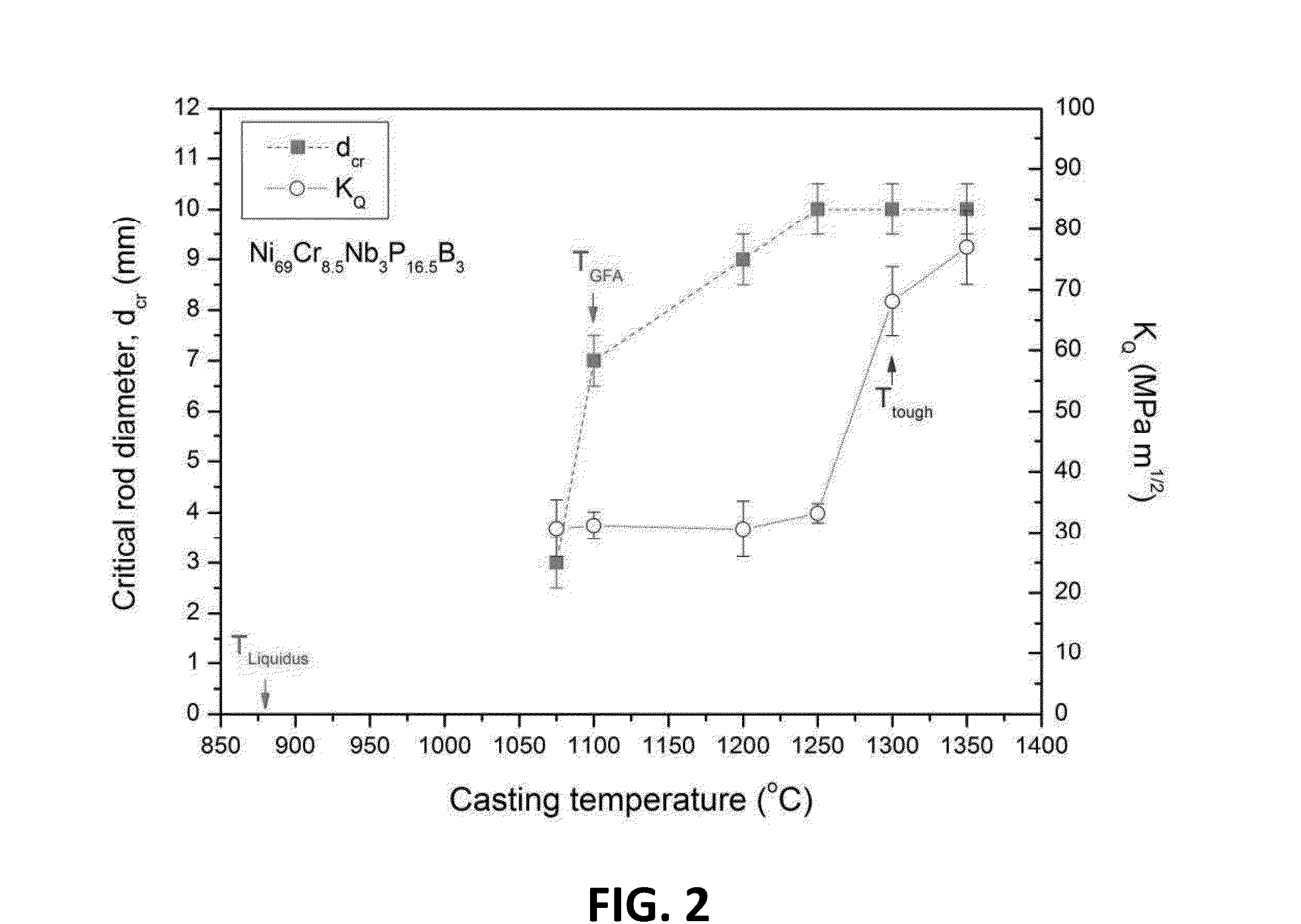
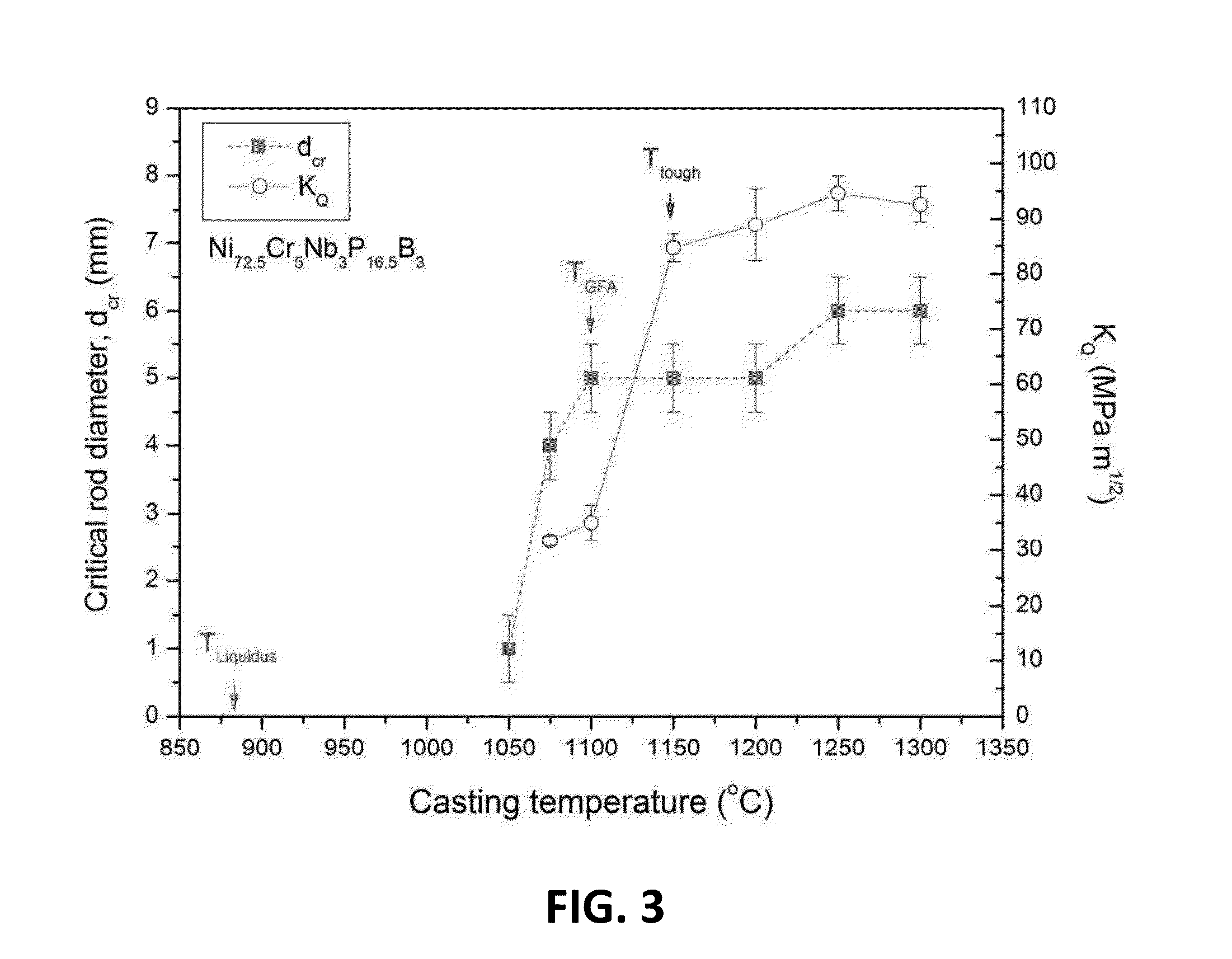
Melt overheating method for improved toughness and glass-forming ability of metallic glasses
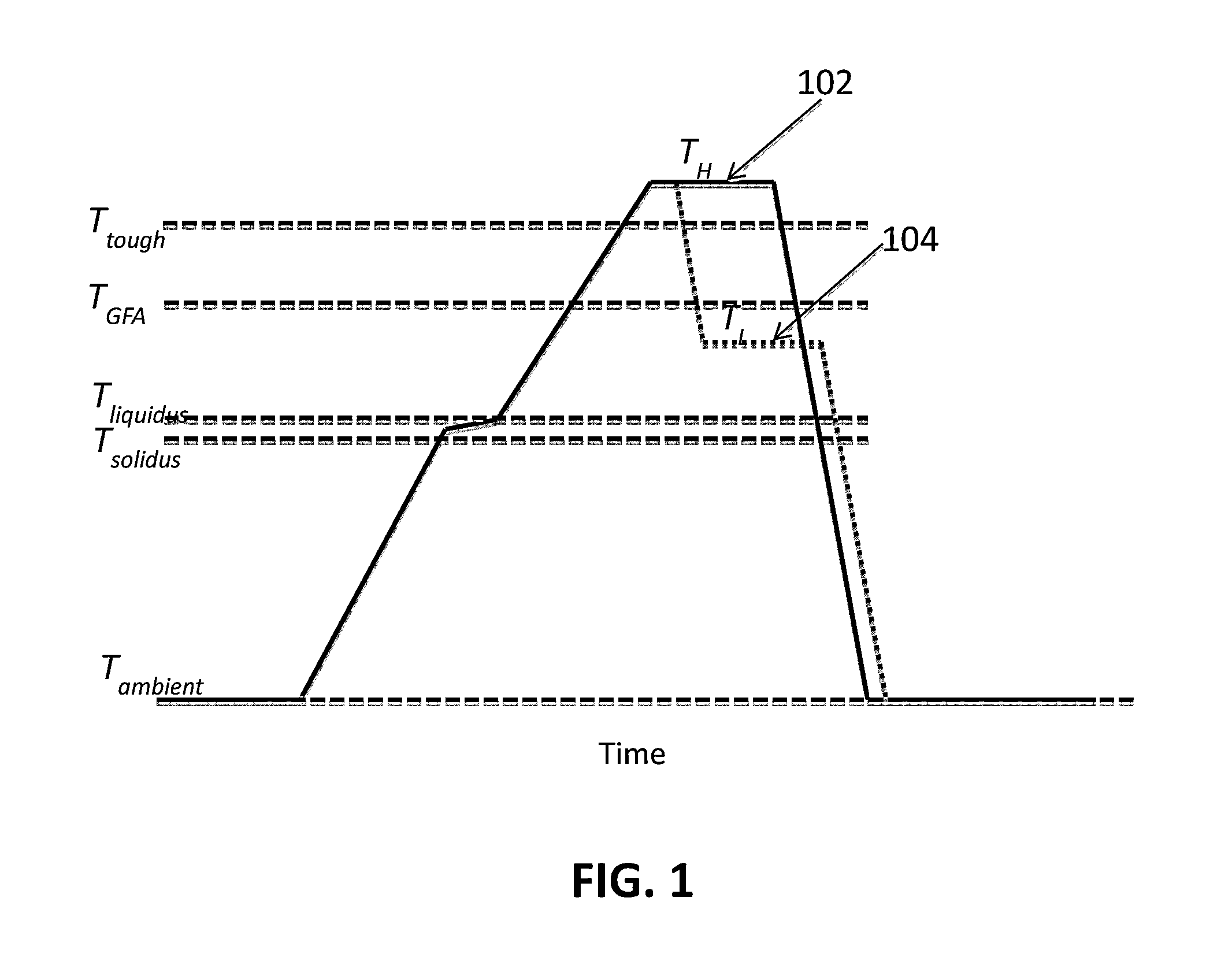
A method of forming a bulk metallic glass is provided. The method includes overheating the alloy melt to a temperature above a threshold temperature, Ttough, associated with the metallic glass demonstrating substantial improvement in toughness compared to the toughness demonstrated in the absence of overheating the melt above Tliquidus, and another threshold temperature, TGFA, associated with the metallic glass demonstrating substantial improvement in glass-forming ability compared to the glass-forming ability demonstrated in the absence of overheating the melt above Tliquidus. After overheating the alloy melt to above Ttough and TGFA, the melt may be cooled and equilibrated to an intermediate temperature below both Ttough and TGFA but above Tliquidus, and subsequently quenched at a high enough rate to form a bulk metallic glass.
Owner:APPLE INC
Rare-earth permanent magnetic powder, bonded magnet, and device comprising the same
ActiveUS20130020527A1High glass forming abilityLow viscosityMaterial nanotechnologySynthetic resin layered productsRare earthAlloy
A rare-earth permanent magnetic powder, a bonded magnet, and a device comprising the bonded magnet are provided. The rare-earth permanent magnetic powder is mainly composed of 7-12 at % of Sm, 0.1-1.5 at % of M, 10-15 at % of N, 0.1-1.5 at % of Si, and Fe as the balance, wherein M is at least one element selected from the group of Be, Cr, Al, Ti, Ga, Nb, Zr, Ta, Mo, and V, and the main phase of the rare-earth permanent magnetic powder is of TbCu7 structure. Element Si is added into the rare-earth permanent magnetic powder for increasing the ability of SmFe alloy to from amorphous structure, and for increasing the wettability of the alloy liquid together with the addition of element M in a certain content, which enables the alloy liquid prone to be injected out of a melting device. The average diameter of the rare-earth permanent magnetic powder is in the range of 10-100 μm, and the rare-earth permanent magnetic powder is composed of nanometer crystals with average grain size of 10-120 nm or amorphous structure
Owner:GRIREM ADVANCED MATERIALS CO LTD
Iron-base bulk amorphous alloy with high glass-forming ability
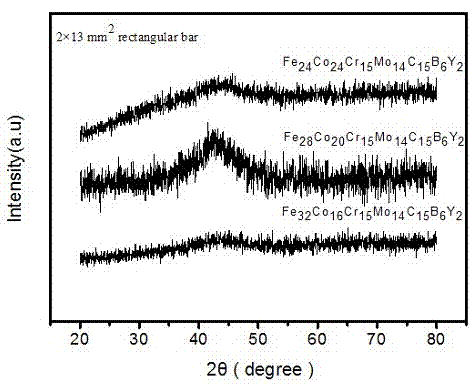
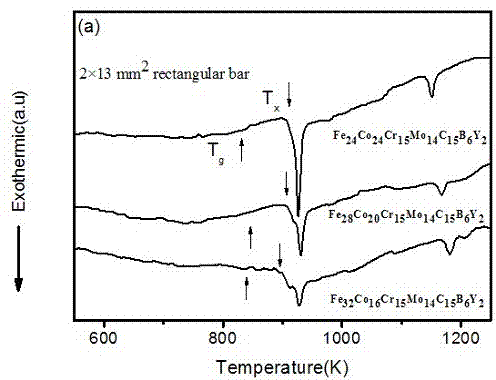
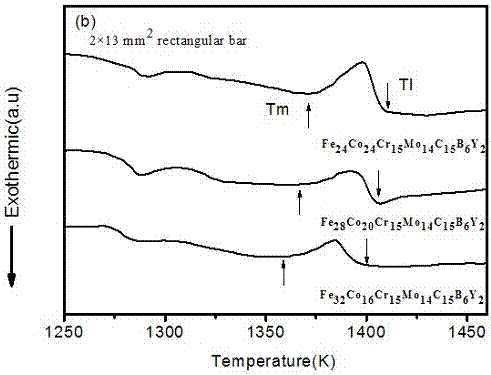
One kind has the high glass to form the ability the hard matrice body crystalless alloy, it involves a kind of crystalless alloy material, specifically designs a kind to have the high glass to form the ability the hard matrice body crystalless alloy. The invention block body crystalless alloy by Fe, Co, Cr, Mo, C, B, Y is composed, various elements atomic percentage is: Fe36~~45%, Co5~~10%, Cr15%, Mo14%, C15%, B6%, Y1~~5%. The invention has the high glass to form the ability, uses the ordinary type mold casting to be allowed to prepare the critical dimension not to be smaller than the 14mm hard matrice body crystalless alloy, has low critical cooling speed 6.5K / s, the high resistant to compression breaking strength approximately is equal to 3500MPa, Gao Weishi degree of hardness Hv approximately is equal to 1230, needs the raw material majority is the industry purity, thus reduced the cost, simultaneously prepares the craft simply, may prepare the critical dimension biggest Fe matrice body crystalless alloy, the industry application potential is very big.
Owner:辽宁峰阁钛业集团有限公司
Iron based amorphous/alumina ceramic composite powder and preparation method and applications thereof
The invention discloses an iron based amorphous / alumina ceramic composite powder and a preparation method and applications thereof. The composite powder comprises following components in percentage byweight: 23.2 to 25.2 wt% of Cr, 3.3 to 4.5 wt% of B, 3.1 to 3.8 wt% of Si, 3.2 to 5.6 wt% of Nb, 1.8 to 2.8 wt% of Ni, 10.3 to 12.3 wt% of Mo, 1.5 to 2.0 wt% of Co, 3.2 to 4.2 wt% of Al2O3, and the balance being Fe. The raw materials are added into an induction cooker and then are heated and melted; the melted metals are atomized and dried, then the powder is sieved; the powder is used to preparea wear-resistant iron based amorphous / alumina ceramic composite protective coating, the coating is prepared by an ultrasonic flame spraying technology, and the obtained coating has the advantages ofhigh hardness, good binding strength, and excellent wear-resistant performance and can be used under severe work conditions such as a high parameter valve sealing surface.
Owner:HOHAI UNIV
Co base Co-Si-B Nb block amorphous alloy
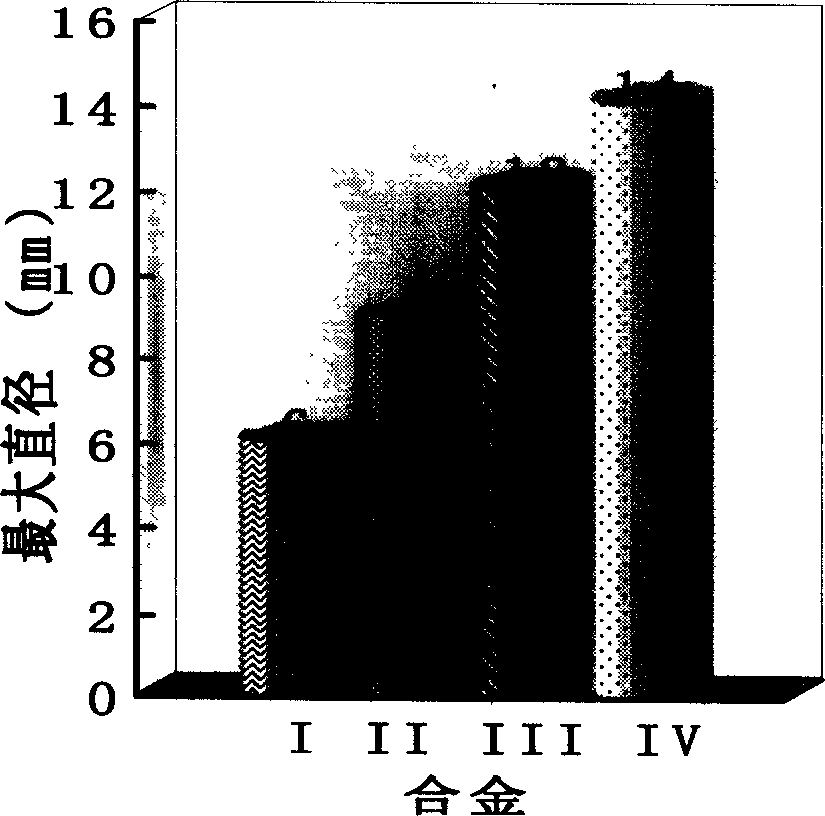
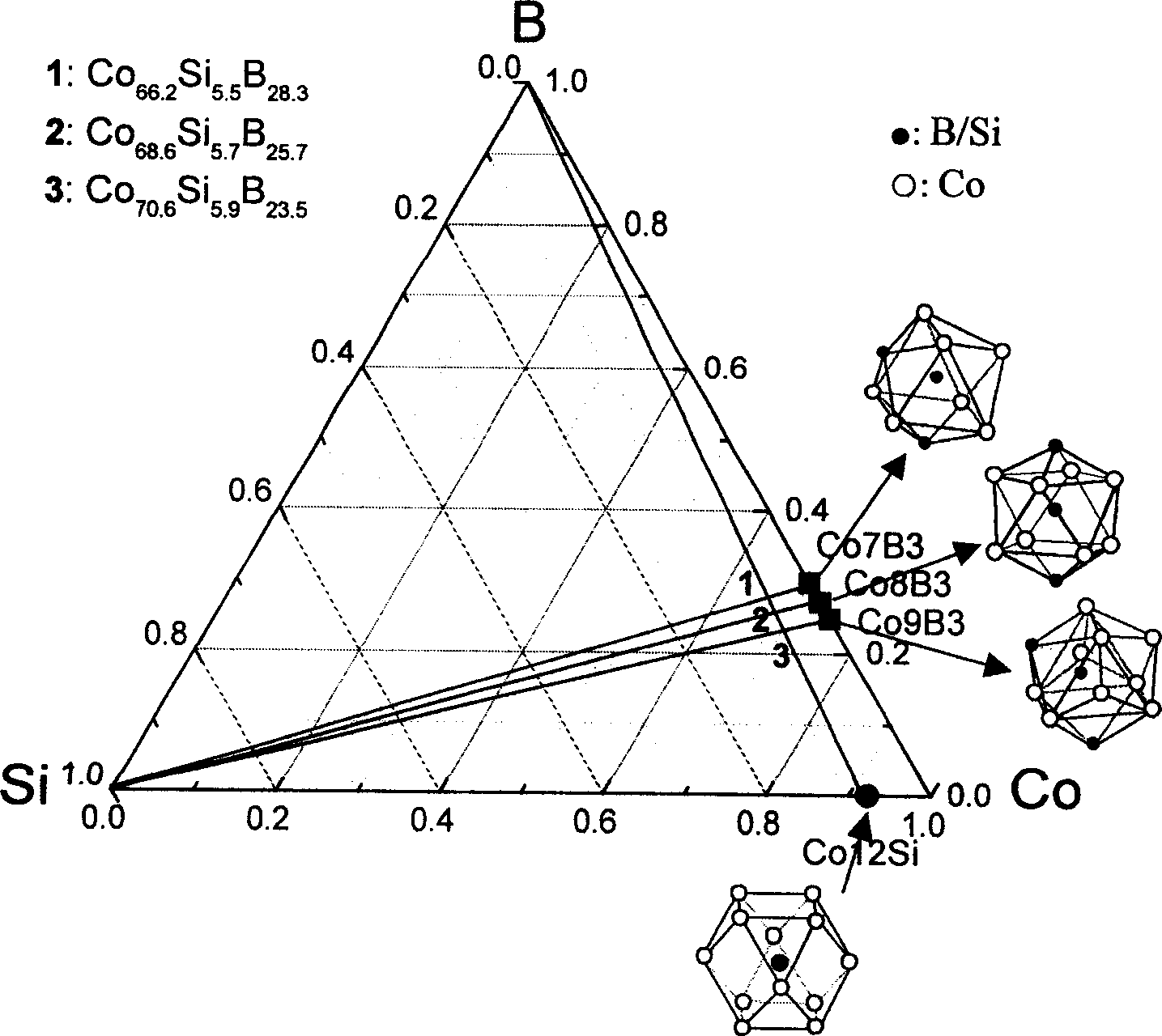
The invention relates to Co radical Co-Si-B-Nb block non-crystal alloy that includes Co, Si, B and Nb elements. It adds the fourth element Nb to Co-Si-B ternary system and taking micro alloying. The constituents range are [(Co12Si)1-xBx]1-yNby, and x=23-29at%, y=3-5at.%; the best block non-crystal ally is (Co68.6Si5.7B25.7)0.96Nb4. The method includes the following processes: mixing, taking copper film negative pressure casting adsorbing, the argon gas pressure is 0.06-0.08MPa, fusion melting current density is 180-220A / cm2, and draught head is 0.03+0.005MPa, the diameter of the block non-crystal is 3mm. The advantages of the invention are that it conquers the random of the constituents and develops Co radical Co-Si-B-Nb block non-crystal alloy. It determines the constituents range and the best constituents.
Owner:DALIAN UNIV OF TECH
Alloy with high glass forming ability and alloy-plated metal material using same
InactiveUS20090246070A1Improve productivityEasy to produceHot-dipping/immersion processesMolten spray coatingMetallic materialsAlloy
An alloy with a high glass forming ability characterized by containing a group of elements A with atomic radii of less than 0.145 nm of a total of 20 to 85 atm %, a group of elements B with atomic radii of 0.145 nm to less than 0.17 nm of a total of 10 to 79.7 atm %, and a group of elements C with atomic radii of 0.17 nm or more of a total of 0.3 to 15 atm %; when the elements with the greatest contents in the group of elements A, group of elements B, and group of elements C are respectively designated as the “element a”, “element b”, and “element c”, by the ratio of the content of the element a in the group of elements A (for example, Zn and / or Al), the ratio of the content of the element b in the group of elements B (for example, Mg), and the ratio of the content of the element c in the group of elements C (for example, Ca) all being 70 atm % or more; and by the liquid forming enthalpy between any two elements selected from the element a, element b, and element c being negative.
Owner:NIPPON STEEL CORP
Iron-based amorphous alloy material with high glass-forming capability
An iron-based amorphous alloy material with high glass-forming capability consists of the elements of Fe, Cr, Mo, C, B, Y and Co, wherein the atomic number ratio of the Fe, Cr, Mo, C, B, Y and Co are (24-32%):(16-24%):15%:14%:15%:6%:2%; the atomic number ratio of the elements of Fe, Cr, Mo, C, B, Y and Co are 24%:24%:15%:14%:15%:6%:2%; the atomic number ratio of the elements of Fe, Cr, Mo, C, B, Y and Co are 28%:20%:15%;14%:15%:6%:2%; and the atomic number ratio of the elements of Fe, Cr, Mo, C, B, Y and Co are 32%:16%:15%:14%:15%;6%:2%. The iron-based amorphous alloy material not only has a simple preparation technology, is low in cost, but also has good amorphous-forming capability and glass-forming capability, and also has mechanical properties with high strength and high hardness.
Owner:NANCHANG HANGKONG UNIVERSITY
Cu-base amorphous alloy
Owner:JAPAN SCI & TECH CORP
Method for preparing amorphous ceramic coating
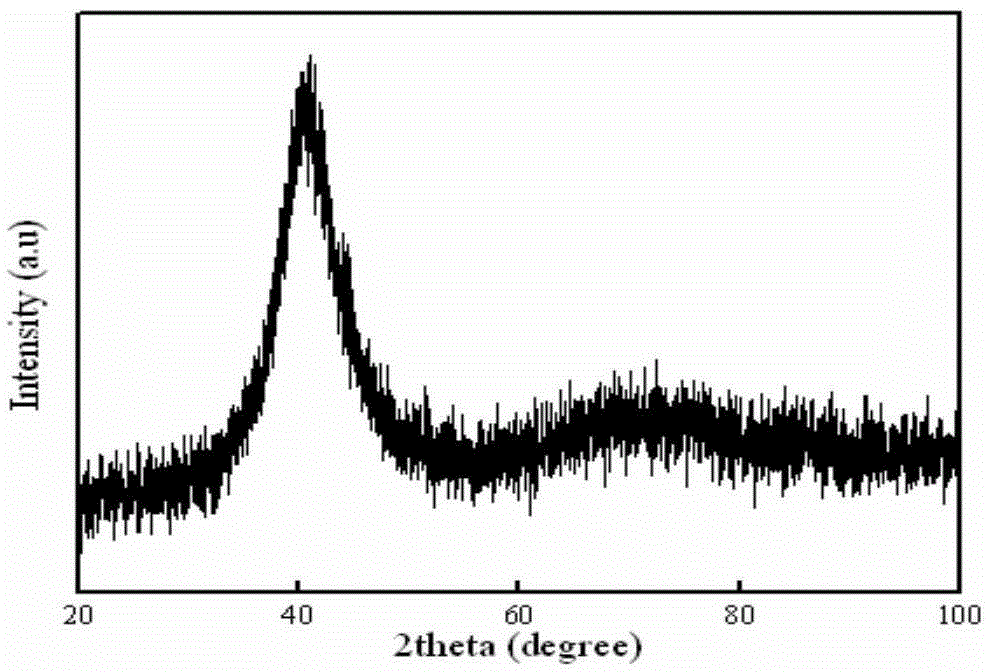
ActiveCN103952695ASolve defects that are prone to cracksHigh glass forming abilityMetallic material coating processesCeramic coatingRare earth
The invention discloses a method for preparing an amorphous ceramic coating. The method comprises the following steps: processing laser cladding into a powder by using a ceramic material, cladding the powder onto a substrate through laser equipment by a laser cladding technology to form the an amorphous ceramic coating with amorphous structure on the main part. The laser cladding ceramic material comprises the following components: a 20%-75% of aluminum oxide, 10%-40% of zirconia and 10%-60% of rare earth oxide. The method provided by the invention improves the glass forming ability, avoids the phenomenon of easy generation of cracks due to rapid melting and rapid cooling in the processing of laser, greatly improves the rate of finished products and reduces the production cost; and the amorphous ceramic coating gains significantly improved toughness and strength and has excellent wear resistance and corrosion resistance.
Owner:DANYANG JUCHEN OPTOELECTRONICS TECH
Block copper-based amorphous alloy and preparation method thereof
The invention relates to a large-block copper-based amorphous alloy and a preparation method thereof. The large-block copper-based amorphous alloy comprises the following components of, by atomic percent, 43-48 at.% of Zr, 5-10 at.% of Al, 0-2 at.% of Sn and the balance Cu. According to the large-block copper-based amorphous alloy and the preparation method thereof, the novel copper-based large-block amorphous alloy with the critical casting diameter of 3 mm in a Cu-Zr-Al system and a Cu-Zr-Al-Sn system is directly determined through combination of thermodynamic calculation and a binary eutectic ratio method; and the generation of a precipitation phase is inhibited by regulating and controlling the binary eutectic ratio, and the large-block amorphous alloy with the critical casting diameter of 5 mm and the large-block amorphous alloy with the critical casting diameter of 7 mm are obtained in the Cu-Zr-Al system and the Cu-Zr-Al-Sn system correspondingly.
Owner:TONGJI UNIV
Ce-Ga-Cu-Al bulk amorphous alloy
The invention discloses Ce-Ga-Cu-Al bulk amorphous alloy which has a structural formula of Ce[70-x]Ga8Cu22Alx, wherein x is atomic percent of Al and is not less than 1 and not more than 6. Compared with corresponding ternary Ce-Ga-Cu bulk amorphous alloy, the Ce-Ga-Cu-Al bulk amorphous alloy has the characteristics that both the glass forming ability and the thermal stability are improved, and the excellent property of relatively low glass-transition temperature of Ce-Ga-Cu alloy is still kept; the Ce-Ga-Cu-Al bulk amorphous alloy is favorable for promoting relatively wide application of Ce amorphous alloy.
Owner:HEFEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com