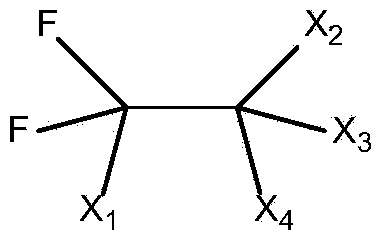
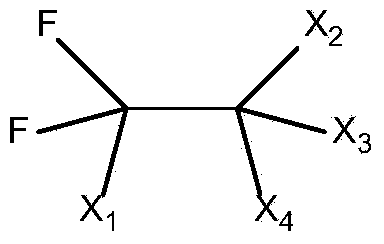
Preparation method of trifluoroacetic acid
A technology of trifluoroacetic acid and trifluoroacetyl fluoride, applied in the preparation of acid halide, preparation of acid halide, preparation of halogenated hydrocarbons, etc., can solve the problems of difficult separation, high cost, and production of many by-products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 4230g of 1,1-difluoro-1,2,2-trichloroethane into a 5L enamel reactor with a reflux condenser, and illuminate with ultraviolet light, the material in the kettle is heated to 40°C, and then Feed chlorine, the chlorine feeding rate is 600g / hour, and the reaction time is 3 hours. The reaction product was degassed and rectified to obtain 3155 g of 1,1-difluorotetrachloroethane with a purity of 99.5%, and the yield was 84.7%.
[0023] Add 3840g of sulfur trioxide and 35g of chlorosulfonic acid in a 5L enamel reactor with a reflux condenser, and drop 3155g of the above-prepared 1,1-difluorotetrachloroethane into the reactor through a conduit for 2 hours After dripping, the temperature in the reactor is controlled at 50°C, and the temperature of the reflux condenser is controlled at 28°C. The gas generated by the reaction passes through concentrated sulfuric acid to absorb unreacted sulfur trioxide, and then obtains 2565g of liquid through compression distillation, which is...
Embodiment 2
[0027] Add 3375g of 1,1-difluoro-1,2-dichloroethane into a 5L enamel reactor with a reflux condenser, and illuminate with ultraviolet light, the temperature of the material in the kettle is raised to 45°C, and then the Chlorine, the chlorine feed rate is 1300g / hour, and the reaction time is 3 hours. The reaction product was degassed and rectified to obtain 2879 g of 1,1-difluorotetrachloroethane with a purity of 99.5%, and the yield was 77.3%.
[0028] Add 3840g of sulfur trioxide and 50g of chlorosulfonic acid in a 5L enamel reactor with a reflux condenser, and drop 2879g of the above-prepared 1,1-difluorotetrachloroethane into the reactor through a conduit for 2 hours After dripping, the temperature in the reactor is controlled at 60°C, and the temperature of the reflux condenser is controlled at 28°C. The gas generated by the reaction passes through concentrated sulfuric acid to absorb unreacted sulfur trioxide, and then obtains 2421.6g of purity 99.3%1 through compression ...
Embodiment 3
[0032] Add 2515g of 1,1-difluoro-1-chloroethane into a 5L enamel reactor with a reflux condenser, and illuminate with ultraviolet light. The feeding rate is 1770g / hour, and the reaction time is 4 hours. The reaction product was degassed and rectified to obtain 2340 g of 1,1-difluorotetrachloroethane with a purity of 99.5%, and the yield was 62.8%.
[0033] Add 3840g of sulfur trioxide and 50g of chlorosulfonic acid in a 5L enamel reactor with a reflux condenser, and drop 2340g of the above-prepared 1,1-difluorotetrachloroethane into the reactor through a conduit for 2 hours After dripping, the temperature in the reactor was controlled at 70°C, and the temperature of the reflux condenser was controlled at 28°C. The gas generated by the reaction passed through concentrated sulfuric acid to absorb unreacted sulfur trioxide, and then obtained 1953.3g of 1953.3g with a purity of 99.0%1 through compression distillation. 1-Difluoro-1-chloroacetyl chloride, yield 83.5%.
[0034] Add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com