Method for catalytic conversion of alkyl cyclohexanol and alkyl cyclohexanone from air oxidized alkyl cyclohexane
A technology of alkyl cyclohexanol and cyclohexane, which is applied in the field of catalytic air oxidation of alkyl cyclohexane to alkyl cyclohexanol and alkyl cyclohexanone, which can solve the problems of high price and achieve the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
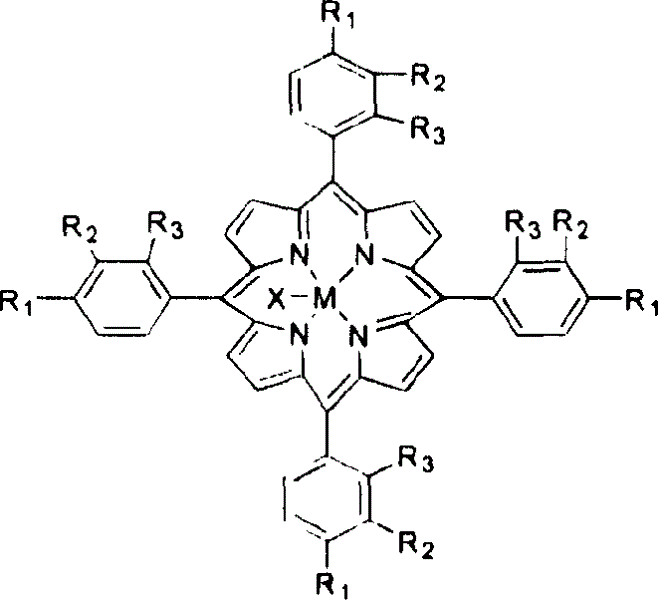
[0018] With 3 mg of metalloporphyrins of general formula (I), R 1 = R 2 = R 3 =CH 3 , M=Mn, add 400ml of methylcyclohexane, and pass through 6atm air. The reactant was stirred at 130° C. for 4 hours, the conversion rate of methylcyclohexane was 18.2%, and the yield of alcohol and ketone in the reaction product was 95%.
Embodiment 2
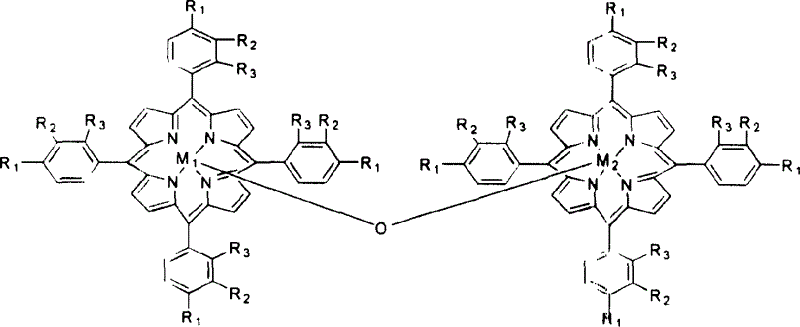
[0020] With 1 mg of metalloporphyrins of general formula (II), R 1 = R 2 = R 3 =Cl,M 1 = M 2 =Fe, added to 500ml 1,4-dimethylcyclohexane, and 8atm air was introduced. The reactant was stirred at 160° C. for 4 hours, the conversion rate of 1,4-methylcyclohexane was 15.5%, and the yield of alcohol and ketone in the reaction product was 92%.
Embodiment 3
[0022] With 4 mg of metalloporphyrins of general formula (I), R 1 = R 2 = R 3 =Cl, M=Fe, and 15mg CoCl 2 Add 500ml of methylcyclohexane, and pass through 10atm air. The reactant was stirred at 140° C. for 3 hours, the conversion rate of methylcyclohexane was 22.5%, and the yield of alcohol and ketone in the reaction product was 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com