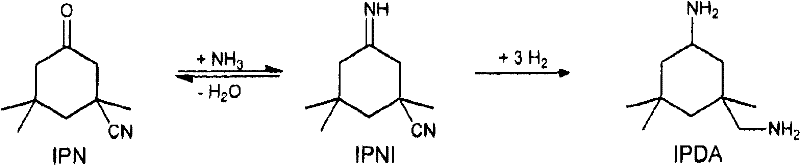
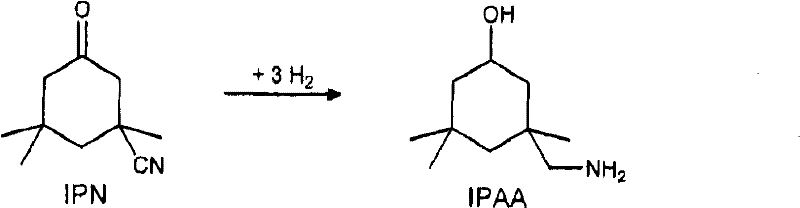
Process for preparing 3-aminomethyl-3,5,5-trimethylcyclohexylamine
A technology of trimethylcyclohexylamine and aminomethyl, which is applied in the field of preparing 3-aminomethyl-3 and can solve problems such as production stoppage and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0223] According to I
[0224] Embodiment, according to the present invention:
[0225] The crude isophorone mixture consisting of isophorone, low-boiling components, high-boiling components as well as water and catalyst produced in the manner described above is removed from the hydrolysis unit, cooled to about 40-60° C. and phase-separated. The comparison is 1 part organic phase, 4 parts aqueous phase. The aqueous phase then still contains 1% by weight of isophorone, which corresponds to approximately 4% by weight of the organic phase. During the subsequent distillation of the waste water, almost the entire isophorone fraction, and up to 75% by weight of water, are evaporated and introduced into the hydrolysis plant.
[0226] When the bottom product of the waste water column is cooled, the remaining 25% by weight of water is recovered by evaporation under reduced pressure.
[0227] For the calculation of 1 ton of isophorone product: increase the production of 4% by weight ...
Embodiment 1
[0261] In the above test parameters, 21.5% IPN ammonia solution when LHSV is 1.8l Lsg l Kat -1 h -1 Amination and hydrogenation. Cobalt catalyst supported on diatomaceous earth was used as catalyst. The pressure in the device was 252 bar. The temperature profile adjusted in the hydrogenation reaction conforms to the adiabatic reaction process, the temperature of the reactor inlet is 70 °C, and the outlet is 115 °C. The mixture leaving the reaction portion was studied by gas chromatography. The composition is given in Table 1.
[0262] Table I:
[0263]
Embodiment 2
[0265] As in Example 1, but after the imidization reactor, an additional 40 g / h of 10% aqueous KCN were metered in. This corresponds to a HCN loading of 1000 ppmw relative to IPN. The results of gas chromatographic analysis of the reaction products are given in Table 2.
[0266] Table 2:
[0267]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com