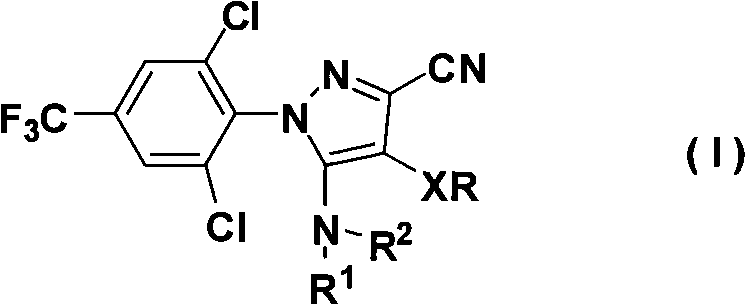
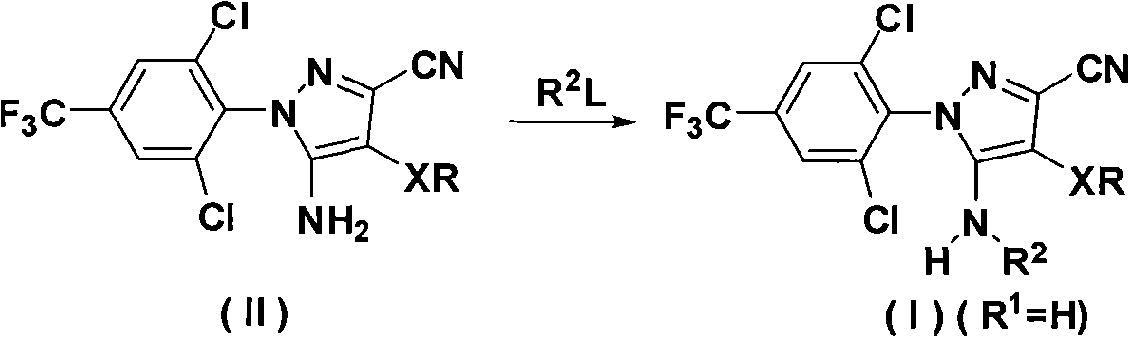
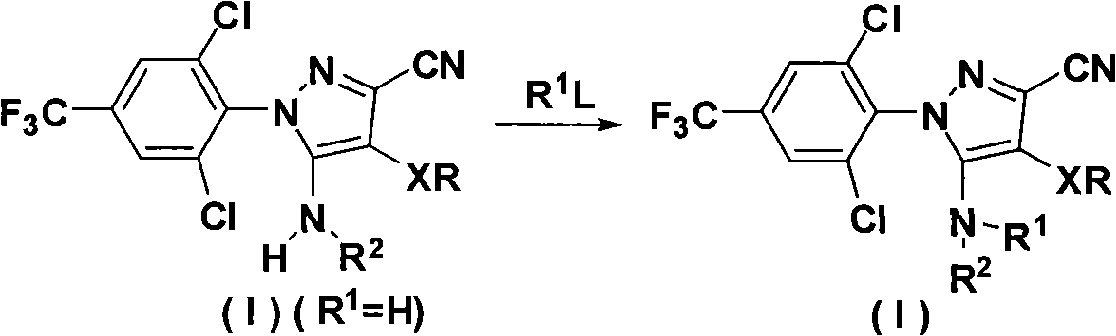
N-benz-3-substituted amino pyrazoles compounds with insecticidal activity
A technology of aminopyrazoles and compounds, applied in the field of N-phenyl-3-substituted aminopyrazoles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation method of compound 01 in Table 1.
[0063] Add 4.6g (10.5mmol) of the compound of formula (II) (X=SO) in Reaction Formula 1 into a three-necked flask with a condenser tube, then add 1.12g (20mmol) KOH and 10ml DMF, and drop in 2.3g ( 21mmol) of 1,3-transdichloropropene, after dropping, the solution turned from light yellow to brownish red, and reacted overnight at room temperature. The reactant was poured into 250ml of water for washing, extracted with ethyl acetate, and dried over anhydrous sodium sulfate. concentrate. Column chromatography (petroleum ether: ethyl acetate = 12:1) gave 2.1 g of a white powdery solid with a content of 95% and a yield of 38.96%. Melting point: 174.3~175.6℃
Embodiment 2
[0065] The preparation method of compound 02 in Table 1.
[0066] Add 4.6g (10.5mmol) of the compound of formula (II) (X=SO) in Reaction Formula 1 into a three-necked flask with a condenser tube, then add 1.12g (20mmol) KOH and 10ml DMF, and drop in 2.3g ( 21mmol) of 2,3-dichloropropene, after dropping, the solution turned from light yellow to deep yellow, and reacted overnight at room temperature. The reactant was poured into 250ml of water for washing, extracted with ethyl acetate, and dried over anhydrous sodium sulfate. concentrate. Column chromatography (petroleum ether: ethyl acetate = 20:1) yielded 2.3 g of off-white powdery solid, content 95.12%, yield 42.72%. Melting point: 168.4~169.5℃
Embodiment 3
[0068] The preparation method of compound 08 in Table 1.
[0069] Add 4.4g (10.5mmol) of the compound of formula (II) (X=S) in Reaction Formula 1 into a three-necked flask with a condenser tube, then add 1.12g (20mmol) KOH and 10ml DMF, and add 2.2g dropwise under stirring at room temperature (20mmol) (E) 1,3-dichloropropene, after dropping, react overnight at room temperature. The reactant was poured into 250ml of water for washing, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and concentrated. Column chromatography (petroleum ether: ethyl acetate = 5:1) gave 2.3 g of a white solid with a content of 95% and a yield of 44.23%. Melting point: 121.5~123.0℃
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com