Organic electroluminescent material containing tertiary aromatic amine structure and preparation method and application thereof
A triarylamine, electroluminescence technology, used in luminescent materials, chemical instruments and methods, circuits, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of Example 1 Compound M101
[0030] The synthesis of step 1, diarylamine compound:
[0031]
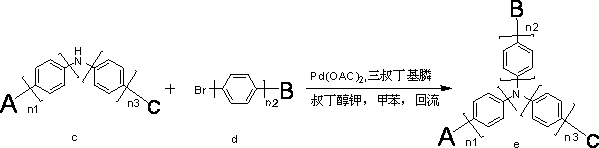
[0032] Under nitrogen protection system, weigh 100mmol (20.9g) of ligand fluorenyl aromatic amine a and 110mmol (17.16g) of aryl halide b into the reaction system, add KOH solid 400mmol (22.4g), add dry dehydrated Toluene 500ml, under nitrogen protection, catalyst CuI 10mmol (1.9g), 1.10-phenanthroline 20mmol (3.6g), under nitrogen protection, reflux reaction for 5 hours, cooling after reaction, suction filtration, concentration, dichloromethane as solvent, Perform column chromatography on silica gel and concentrate to obtain 26.22 g of an off-white diamine product with a yield of more than 92% and an HPLC purity of more than 98%. It can be used directly for the next reaction.
[0033] Step 2, the synthesis of triarylamine compound (M101):
[0034]
[0035] Weigh 50mmol (14.25g) of diarylamine compound, 55mmol (17.66g) of carbazolyl halide, 75mmol (8.4g) of p...
Embodiment 2
[0037] Synthesis of Example 2 Compound M106
[0038] The synthesis of step 1, diarylamine compound:
[0039]
[0040] Under nitrogen protection system, weigh 100mmol (25.9g) of ligand fluorenyl aromatic amine a and 110mmol (28.60g) of aryl halide b into the reaction system, add KOH solid 400mmol (22.4g), add dry dehydrated Toluene 500ml, under nitrogen protection, catalyst CuI 10mmol (1.9g), 1.10-phenanthroline 20mmol (3.6g), under nitrogen protection, reflux reaction for 10 hours, cooling after reaction, suction filtration, concentration, dichloromethane as solvent, Perform column chromatography on silica gel and concentrate to obtain 39.51 g of off-white diamine product with a yield of 90% and a purity of more than 98% by HPLC. It can be used directly for the next reaction.
[0041] Step 2, the synthesis of triarylamine compound (M106):
[0042]
[0043] Weigh 50mmol (21.95g) of diarylamine compound, 55mmol (17.66g) of carbazolyl halide, 75mmol (8.4g) of potassium t...
Embodiment 3
[0046] Example 3 Synthesis of compound M109
[0047] The synthesis of step 1, diarylamine compound:
[0048]
[0049] Under nitrogen protection system, weigh 100mmol (28.5g) of ligand fluorenyl aromatic amine a and 110mmol (25.52g) of aryl halide b into the reaction system, add KOH solid 400mmol (22.4g), add dry dehydrated Toluene 500ml, under nitrogen protection, catalyst CuI 10mmol (1.9g), 1.10-phenanthroline 20mmol (3.6g), under nitrogen protection, reflux reaction for 8 hours, cooling after reaction, suction filtration, concentration, dichloromethane as solvent, Perform column chromatography on silica gel and concentrate to obtain 20.11 g of off-white diamine product with a yield of 92% and an HPLC purity greater than 98%. It can be used directly for the next reaction.
[0050] Step 2, the synthesis of triarylamine compound (M109):
[0051]
[0052] Weigh 50mmol (21.85g) of diarylamine compound, 55mmol (21.84g) of carbazolyl halide, 75mmol (8.4g) of potassium tert...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com