Sabin strain poliovirus type III monoclonal antibody and application thereof
A technology for polio and monoclonal antibodies, applied in the field of immunology, can solve the problems of lack of vaccines and test results that cannot truly reflect the type III immunogenicity of type III antigen vaccines, and achieve good virus-specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Sabin strain poliovirus type III immunogen preparation and animal immunization
[0031](1) Take the Vero cell working cell bank and culture it at 36.5±0.5°C after recovery until the cell concentration is 0.1-10×10 6 cells / ml, the virus was inoculated.
[0032] (2) Inoculate Vero cells with working seeds prepared from sabin strain polio type III virus derived from Intravacc at MOI=10-0.05, and culture at 32.5±0.5°C.
[0033] (3) The virus was cultured for 2-4 days, and the cell supernatant was harvested, which was the poliovirus Sabin strain type III virus harvest liquid.
[0034] (4) Type III harvest liquid is clarified and concentrated more than 5 times with ultrafiltration membrane bag.
[0035] (5) Then carry out molecular sieve chromatography and ion exchange chromatography, the monitoring wavelength is 280nm, collect the eluate and flow-through respectively to obtain the purified solution, and obtain the type III vaccine stock solution after formaldehyd...
Embodiment 2
[0037] Example 2 Cell Fusion and Strain Construction
[0038] (1) Recover and culture the SP2 / 0 cell line before cell fusion, expand the culture 3 days before fusion, remove RPMI 1640 cell culture medium (Gibco) 1 day before fusion, and add culture medium again to prepare SP2 / 0 cells.
[0039] (2) The immunized mice were sacrificed, and the mouse splenocyte suspension was prepared according to conventional methods.
[0040] (3) Add an appropriate amount of incomplete IMDM culture medium (Gibco) according to the counting results of splenocytes and SP2 / 0 cells, shake and mix the SP2 / 0 cells, and pipette the splenocytes evenly. Then the splenocytes and SP2 / 0 cells were mixed in a 50ml centrifuge tube at a ratio of 1:2 to 10:1, and mixed well.
[0041] (4) Add incomplete IMDM culture medium to 50ml, centrifuge for 5-10 minutes, and pour out the supernatant. Lightly tap the bottom of the fusion tube to loosen and evenly precipitate the cells, and place the centrifuge tube in a 37...
Embodiment 3
[0049] Example 3 Monoclonal Antibody Cell Line Ascites Preparation and Antibody Titer Detection
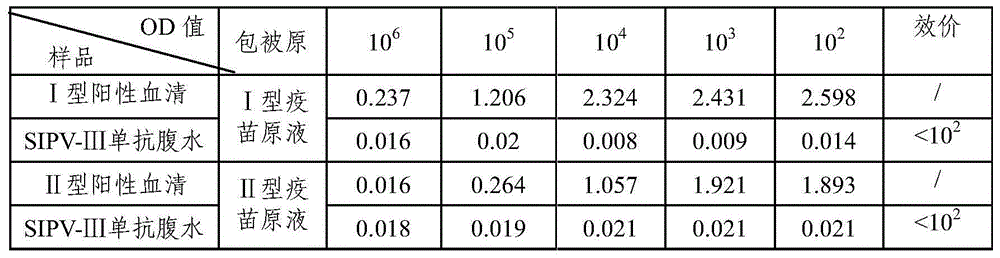
[0050] Resuscitate the frozen hybridoma cells obtained in Example 2 according to conventional methods, and cultivate them. When the cells cover more than 50% of the bottom of the 25ml cell culture bottle, BALB / c mice can be inoculated intraperitoneally according to conventional methods, and the ascites can be collected regularly. SIPV-Ⅲ.
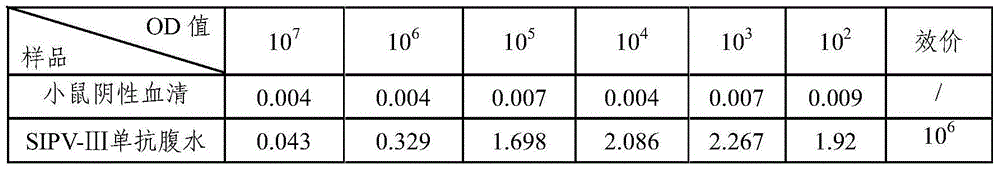
[0051] Poliomyelitis type III vaccine stock solution was diluted with 0.01M PBS1:20, 100μl / well was coated with an enzyme-titer plate, and left overnight at 2-8°C to detect the antibody titer of poliomyelitis type III ascites SIPV-Ⅲ, and the antibody titer was 10 6 , higher potency. The results are shown in Table 1.
[0052] Table 1 Antibody Titer Test Results
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com