Human mycoplasma pneumoniae monoclonal antibody and its application
A technology of mycoplasma pneumoniae and monoclonal antibody, which is applied in the field of immunology and vaccinology, can solve the problems of low positive separation rate, high nutritional requirements, complicated operation, etc., and achieve the effect of large application prospect, strong affinity and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 immunogen preparation and animal immunization
[0037] (1) Cultivate 20L of mycoplasma arginini (bacteria are purchased from ATCC, product number 15531) with the mycoplasma arginini broth culture medium that contains 20% calf serum, and formaldehyde in 1:2000 to the harvesting liquid of mycoplasma pneumoniae Inactivate at 37°C for 120h.
[0038] (2) Concentration of harvested solution of Mycoplasma pneumoniae: the inactivated solution of Mycoplasma pneumoniae was subjected to 300KD 0.1m 2 Concentrate by membrane bag ultrafiltration to 700ml.
[0039] (3) Centrifuge the human Mycoplasma pneumoniae concentrate with 30% and 55% sucrose at 30,000 r / min for 12 hours, and use 0.01mol / L PBS to wash and desugar the human Mycoplasma pneumoniae obtained by centrifugation.
[0040] (4) The protein content of Mycoplasma pneumoniae measured by the Lowry method was 2.7 mg / ml, and the protein concentration was diluted to 500 μg / ml.
[0041] (5) 1 mL of the purified Mycop...
Embodiment 2
[0043] Example 2 Cell Fusion and Strain Construction
[0044] (1) Preparation of myeloma cells: resuscitate and culture SP2 / 0 cell line two weeks before cell fusion, expand culture 3 days before fusion, and change medium of SP2 / 0 cells 1 day before fusion.
[0045] (2) Spleen cell preparation: sacrifice the mice for animal immunization, and prepare the mouse spleen cell suspension according to the conventional method.
[0046] (3) Add an appropriate amount of serum-free 1640 culture medium to splenocytes and myeloma cell SP2 / 0 according to the counting results, shake and mix SP2 / 0 cells, and pipette splenocytes evenly.
[0047] (4) Mix splenocytes and SP2 / 0 cells in a 50ml centrifuge tube at a ratio of 2:1 to 5:1, and mix well.
[0048] (5) Add serum-free 1640 culture medium to 50ml, centrifuge at 1500rpm for 5min, pour out the supernatant as much as possible, and use a pipette to suck up the liquid from the nozzle.
[0049] (6) Lightly tap the bottom of the fusion tube to l...
Embodiment 3
[0060] Example 3 Monoclonal Antibody Cell Line Ascites Preparation and Antibody Titer Detection
[0061] The frozen hybridoma cell MP-H8 obtained in Example 2 was revived according to conventional methods, cultured, and the cell growth status was observed under a microscope, 175cm 2 The cell culture flasks were inoculated when the cells covered 70%-80% of the bottom of the flasks; BALB / c female mice were inoculated intraperitoneally according to conventional methods, and ascites were collected regularly.
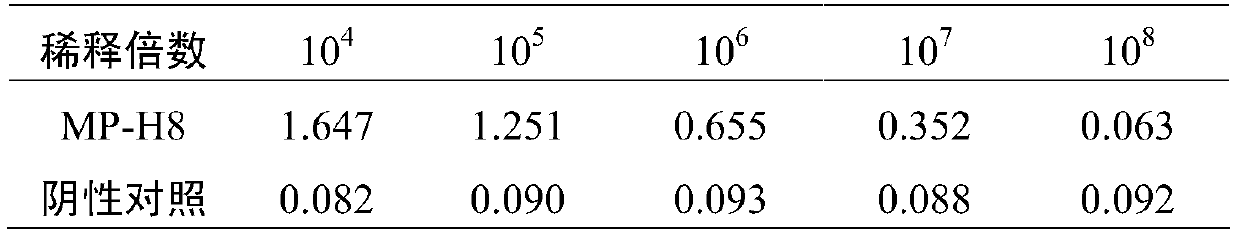
[0062] Ascites identification of human Mycoplasma pneumoniae monoclonal antibody: the titer of antibody was detected by indirect ELISA method. Antibody titer test results: the antibody titer of the ascites was detected after the human Mycoplasma pneumoniae 1 μg / ml was coated overnight, and the antibody titer was 10 7 .
[0063] Table 1 Antibody Titer Test Results
[0064]
[0065] Purification of human mycoplasma pneumoniae monoclonal antibody:
[0066] The ascites wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com