Immunologic diagnosis kit for detecting dengue virus NS1 antigen and application thereof
A technology for dengue virus and immunodiagnosis, applied in the field of medicine, can solve the problems of unfavorable clinical practice application, difficult to repeat standardization, low virus content, etc., and achieve the effect of overcoming difficulty in repeating, reducing missed detection, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] (1) Preparation of immune antigen
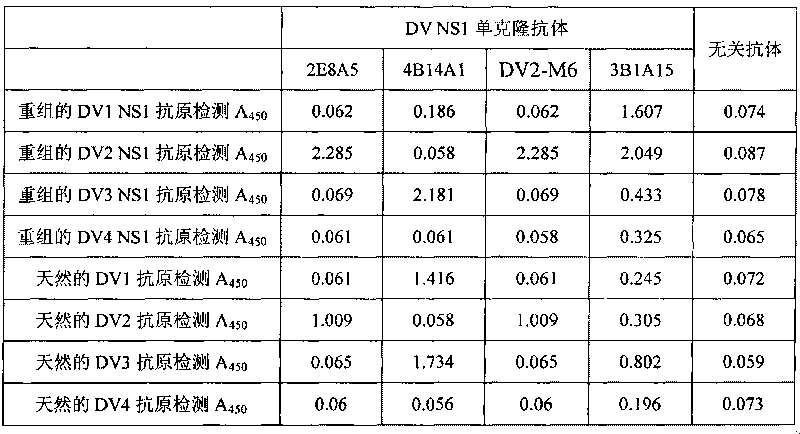
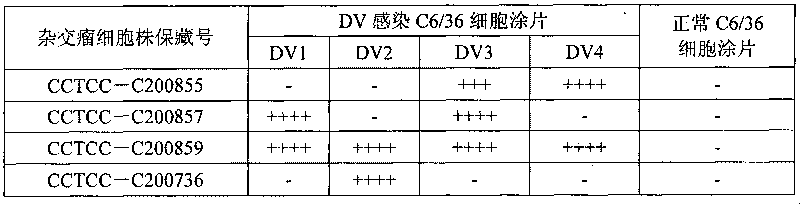
[0017] The immunogen used to prepare the monoclonal antibody in the present invention is gene recombinant DV2NS1, DV3NS1, DV4NS1 protein and inactivated natural virus antigen. The genetically recombinant DVNS1 protein is prepared by an engineering strain carrying the DVNS1 gene, and its preparation is carried out according to a conventional method, and the NS1 antigen is obtained by purifying it with nickel primary nitrogen triacetate metal affinity chromatography, and the detailed preparation method Refer to the user manual. After NS1 protein was purified, it was quantified with Coomassie protein assay reagent (PIERCE, Cat, No. ED62976). The results of Western blot identification of the purified recombinant protein showed that the mouse anti-his mAb had a specific reaction band at a molecular weight of about 45KDa, which was consistent with the predicted molecular weight of DVNS1. The inactivated natural viral antigens are obtained...
example 1
[0043] (1) Preparation of reagents used
[0044] a. Sample treatment solution: composed of sample treatment solution A and sample treatment solution B, wherein A is a 1.5M glycine solution (PH2.8), and B is a 1.5M Tris-Hcl solution (PH9.7);
[0045] b. Concentrated lotion: 20×PBS containing 2% Tween-20, that is, 1L solution contains 4.56g NaH2PO4, 58.02gNa2HPO4.12H 2 O, 175.3g NaCl, 15 pounds of 20min after autoclaving, add 20ml Tween-20 and stir well, dilute 20 times when used;
[0046] c. Positive control: 1:1000 dilution of recombinant DVNS1 protein;
[0047]d. Negative control: 10mM PH7.4PBS containing 0.1% Tween-2, its preparation method is: 4.56g NaH 2 PO4, 58.02gNa 2 HPO4·12H 2 O, 175.3g NaCl dissolved in water and quantified to 1L, autoclaved at 15 lbs pressure for 20min, 0.1% Tween-20 was added after 20-fold dilution;
[0048] e. Chromogenic solution: It is composed of chromogenic solution A and B. When using, take the two equal amounts and mix them well. Wherei...
example 2
[0088] 1. The kit of the present invention consists of the following reagents:
[0089] (1) Microwell reaction plates coated with antibodies 2E8A5, DV2-M6 and 4B14A1;
[0090] (2) Sample treatment solution: composed of sample treatment solution A and sample treatment solution B, wherein A is a 1.5M glycine solution (PH2.8), and B is a 1.5M Tris-Hcl solution (PH9.7);
[0091] (3) Biotin-labeled monoclonal antibody 3B1A15;
[0092] (4) horseradish peroxidase-labeled avidin, purchased from Zymed Company;
[0093] (5) Concentrated lotion: 20×PBS containing 2% Tween-20, that is, 4.56g NaH in 1L solution 2 PO 4 , 58.02gNa 2 HPO 4 .12H 2 O, 175.3g NaCl, 15 pounds of 20min after autoclaving, add 20ml Tween-20 and stir well, dilute 20 times when used;
[0094] (6) Positive control: 1:1000 dilution of DVNS1 antigen;
[0095] (7) Negative control: 10mM PH7.4PBS containing 0.1% Tween-20, that is, 4.56g NaH in 1L solution 2 PO 4 , 58.02gNa 2 HPO 4 .12H 2 O, 175.3g NaCl, 15 pound...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com