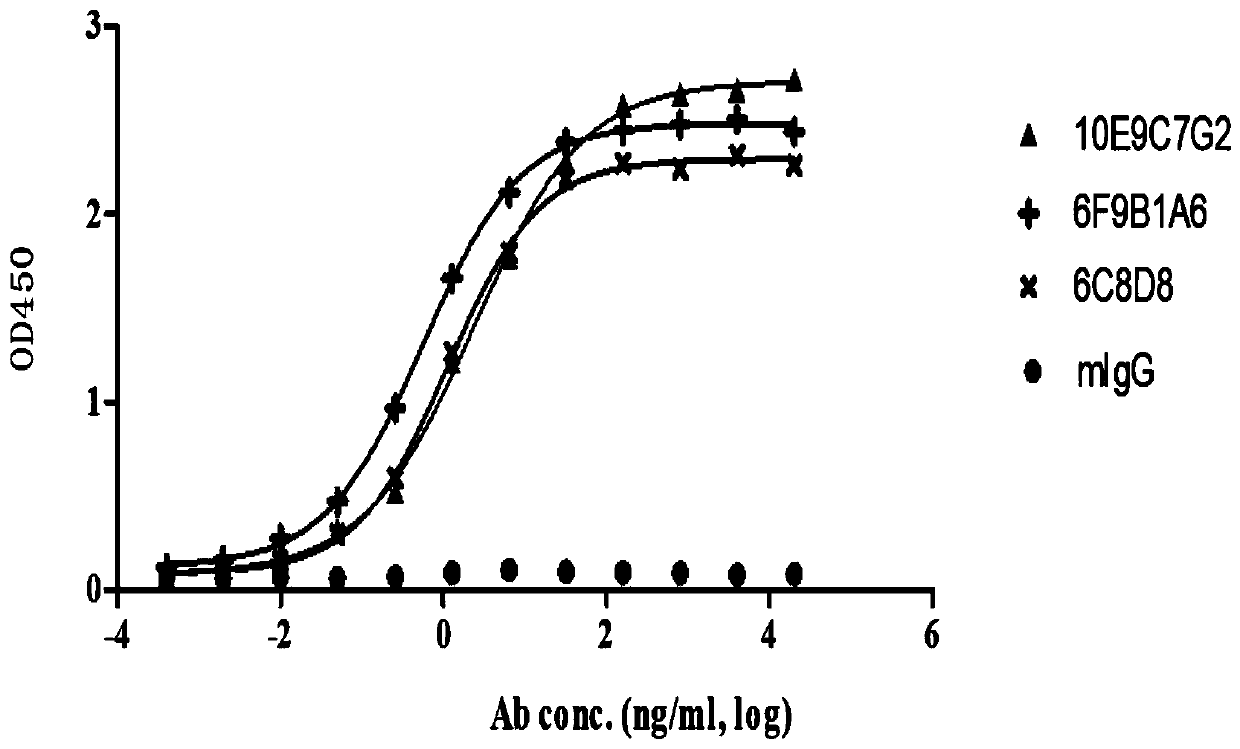
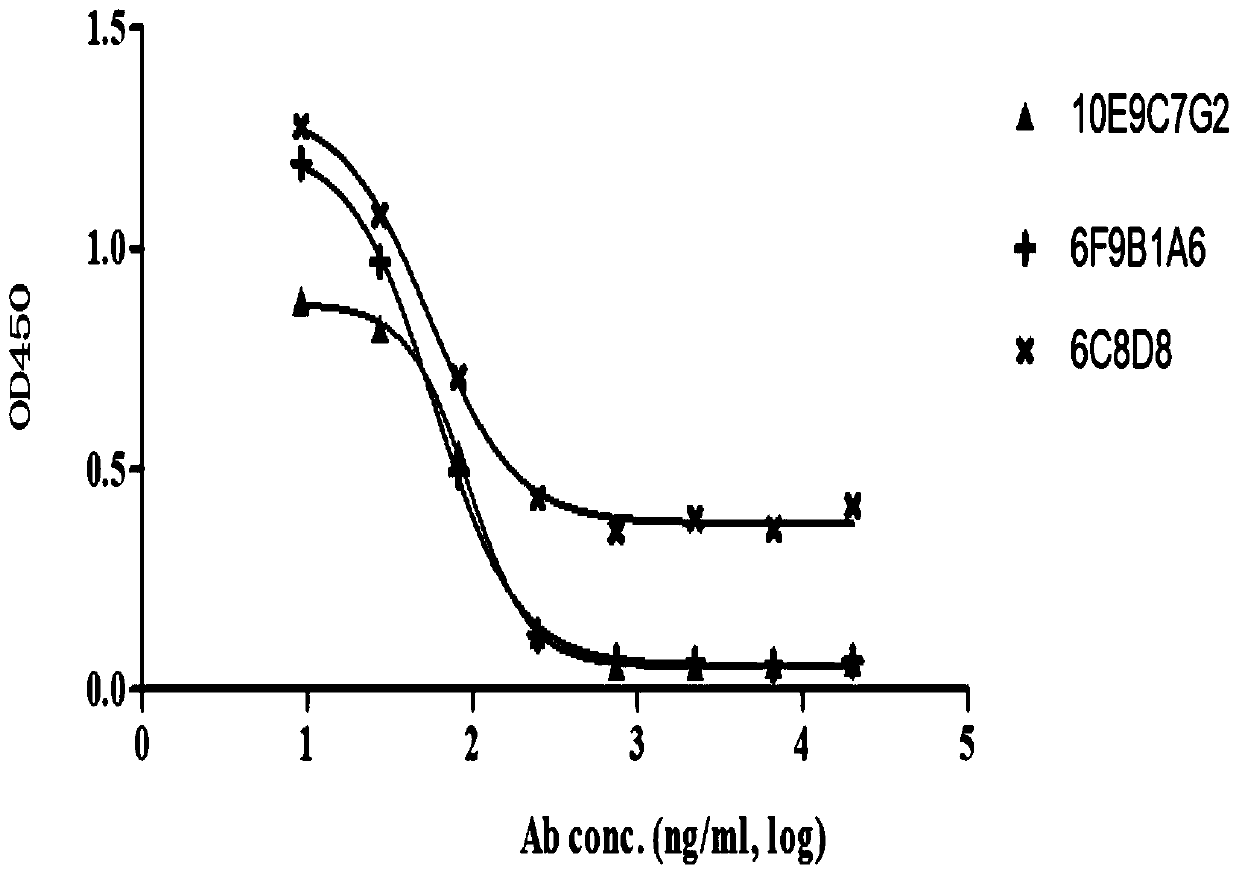
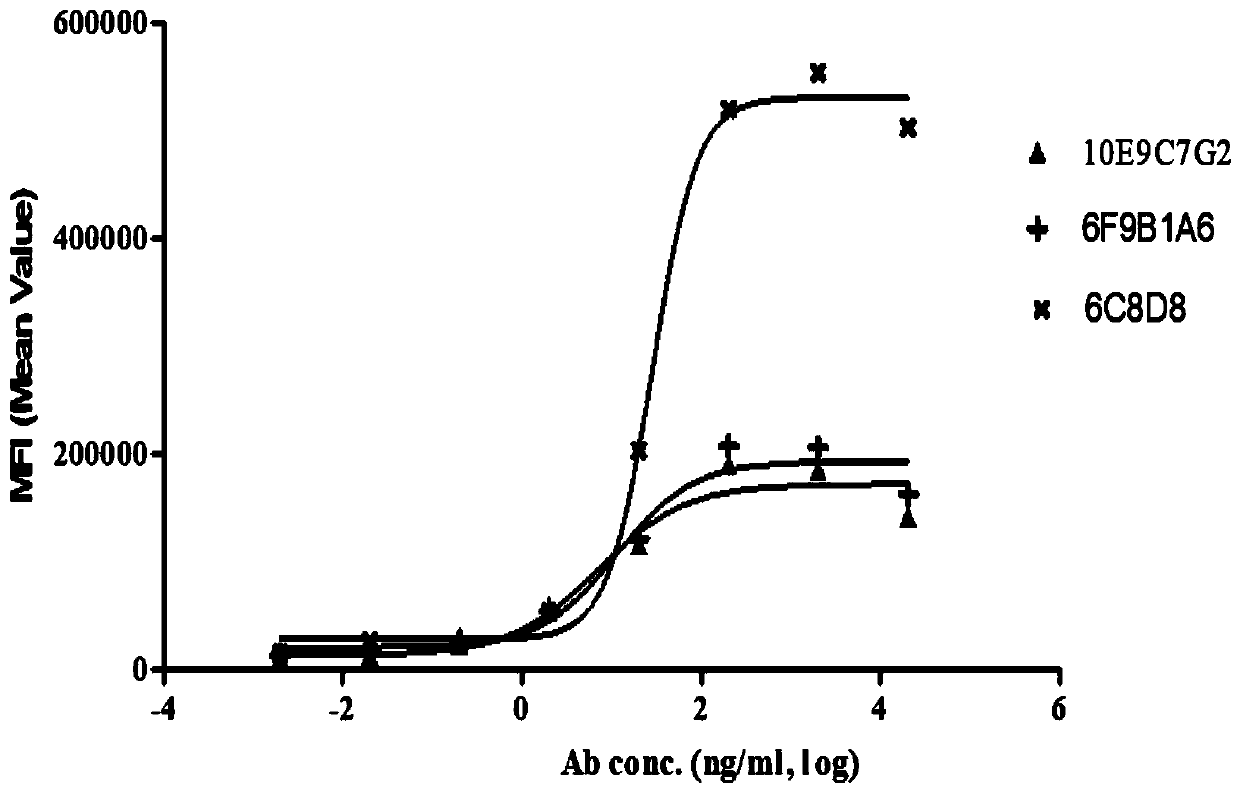
Group of PD-L1-resisting monoclonal antibodies and medical application thereof
A PD-L1, monoclonal antibody technology, applied in the field of tumor and immunology drugs, can solve the problem of low tumor suppression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 - Construction of mouse hybridoma antibody
[0037] The construction of mouse hybridoma antibody includes the following 6 steps.
[0038] (1) Mouse immunization and fusion of hybridoma cells. Human PD-L1-ECD-mFc fusion protein was used as antigen, fully emulsified with an equal volume of complete Freund's adjuvant (Sigma, Cat No: F5581), and subcutaneously immunized 6-8 week-old Balb / c mice (purchased from Zhaoyan (Suzhou) New Drug Research Center Co., Ltd.), the antigen immunization dose was 20 μg / monkey. Subsequently, mice were subcutaneously immunized three times with the same dose of antigen fully emulsified with incomplete Freund's adjuvant (Sigma, Cat No: F5506) every 2 weeks. After three immunizations, the serum titer of the mice was determined, and a booster immunization was carried out through the abdominal cavity 3 days before the fusion. Using PEG Hybri-Max (Sigma, CatNo:7181) as a fusion agent, mouse spleen cells and SP2 / 0 cells were mixed in a ...
Embodiment 2
[0057] Example 2 - Cloning of PD-L1 Antibody Variable Region Gene
[0058] The PD-L1 monoclonal hybridoma cell line was lysed with TRIzon (Cwbiotech, Cat No: CW0580), and the total RNA of the hybridoma cells was extracted. The RNA of hybridoma cells was reverse-transcribed into cDNA using HiFi Script cDNA Synthesis Kit (Cwbiotech, Cat No: CW2569). Using cDNA as a template, amplify the heavy chain of the antibody by PCR method (Kettleborough et al. (1993) Eur J Immunology 23:206-211; Strebe et al. (2010) Antibody Engineering 1:3-14) with degenerate primers and light chain variable region genes. After the PCR amplification product was connected to the T / A carrier, the DH5a competent cells were transformed, plated and cultured overnight at 37°C. Pick a single clone from the culture plate, expand the culture, extract the plasmid, and determine the gene sequence of the antibody. According to the gene sequence of the antibody, its complementary determinants (CDR) and framework re...
Embodiment 3
[0062] Example 3 - Humanization of murine PD-L1 antibodies 6C8D8, 6F9B1A6 and 10E9C7G2
[0063] (1) The humanization of PD-L1 antibody was carried out by the complementary determinant grafting method. First, the human germline antibody sequences with the highest homology to the light and heavy chain variable regions of the murine 6C8D8, 6F9B1A6 and 10E9C7G2 antibodies were searched in the IMGT database. The germline selected for the humanization of the light chain variable region of the 6C8D8 antibody is IGKV6-21*02, and the humanization of the heavy chain variable region is IGHV2-5*01. The germline selected for the humanization of the light chain variable region of the 6F9B1A6 antibody For IGKV1-9*01, IGHV1-69*08 was selected for the humanization of the heavy chain variable region. The germline selected for the humanization of the light chain variable region of the 10E9C7G2 antibody was IGKV1-39*01, and the heavy chain variable region was humanized Select IGHV6-1*01.
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com