Monoclonal antibody of avian leukosis virus subgroup J surface protein and preparation method thereof
A technology of avian leukosis virus and monoclonal antibody, which is applied in the interdisciplinary field of molecular immunology and virology, can solve problems such as false positives, false negatives, and inaccurate test results, and achieve low-cost results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Cloning and sequencing of ALV-J gp85 gene
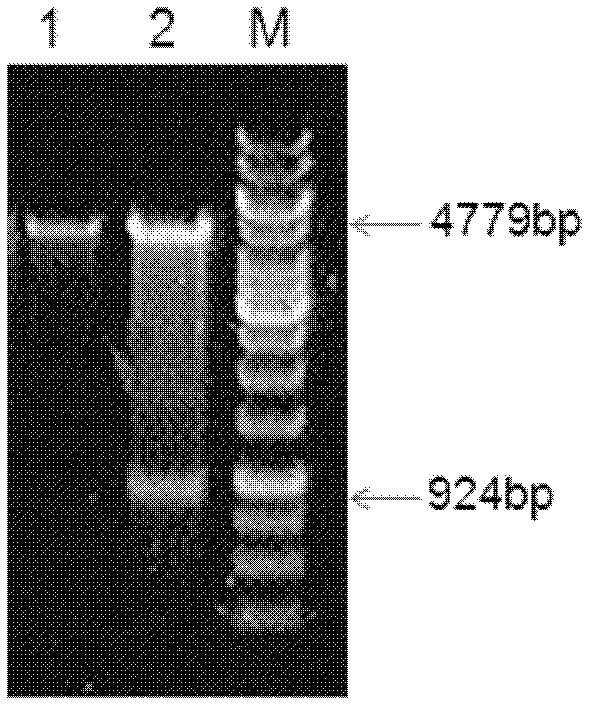
[0072] The sequences at both ends of the envelope glycoprotein gene of ALV-J Chinese strain WS0705 provirus genome DNA gp85 were used as primers. The forward primer is 5'-CTGGATCCATGGGAGTTCATCTATTGCAACACCCAG-3', as shown in SEQ ID NO.2, containing the BamHI restriction site; the reverse primer is 5'-TACTGCAGT TAG CGC CTG CTA CGG TGG TGA CC-3', as shown in SEQ ID NO. .3, containing PstI enzyme cutting site. The PCR reaction conditions were: 95°C for 4 min, fully denatured and then entered the cycle system: 95°C for 30 s, 59°C for 1 min, 72°C for 2 min, a total of 30 cycles; then extended at 72°C for 10 min. The PCR product was purified using a DNA purification kit, and the length of the PCR product was detected to be 924 base pairs by 1% agarose gel electrophoresis. figure 1 As shown, its gene sequence is shown in SEQ ID NO.1, and the PCR product was purified using a DNA purification kit.
[0073] The pProEXHTb vector and...
Embodiment 2
[0077] Expression and purification of His-gp85 protein
[0078] Inoculate the BL21 bacteria containing the recombinant plasmid in the LB solid medium containing ampicillin (Amp), cultivate at 37°C, pick a single colony and inoculate it in the LB liquid medium containing Amp (final concentration: 50 μg / ml), at 37°C Shake (200rpm), cultivate to OD600=0.6, add IPTG to a final concentration of 1mmol / L, continue to cultivate at 37°C for 3h, and set BL21 bacteria transformed with uninduced recombinant plasmids as a control.
[0079] SDS-PAGE analysis showed that the target protein His-gp85 was expressed in the form of inclusion bodies, and the detection steps were as follows:
[0080] Collect the cultivated engineering bacteria liquid, centrifuge at 5000rpm for 5min, and discard the supernatant; weigh 30g of the collected wet bacteria into a 500ml beaker, add 300ml of resuspension, add the rotor and stir on a magnetic stirrer for 20min to make the bacteria Disperse evenly; place ...
Embodiment 3
[0107] animal immunity
[0108] The 6-week-old Balb / C female mice were immunized with the above-mentioned purified His-gp85 fusion protein, the immunization dose was 40ug per mouse, and the immunization method was intraperitoneal injection, and the immunization was done four times in total.
[0109] For the first immunization, use the above protein to emulsify the antigen with the same amount of Freund's complete adjuvant, and perform the second immunization two weeks later. During the immunization, emulsify the inactivated antigen with the same amount of Freund's incomplete adjuvant, and perform three immunizations two weeks later , The immunization method and dose are the same as the second immunization. One week after the three times of immunization, blood was collected from the orbit of the mice to measure its titer, and the serum antibody titer was monitored. Two weeks after the third immunization, the mouse with the highest titer was selected, and the antigen without ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com