Two-stage type microneedle array patch capable of simultaneously realizing BCG vaccine inoculation and diagnosis, and preparation method thereof
A microneedle array and needle array technology, applied in the field of biomedical engineering, can solve problems such as unbearable pain, cold chain storage and transportation, and high risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
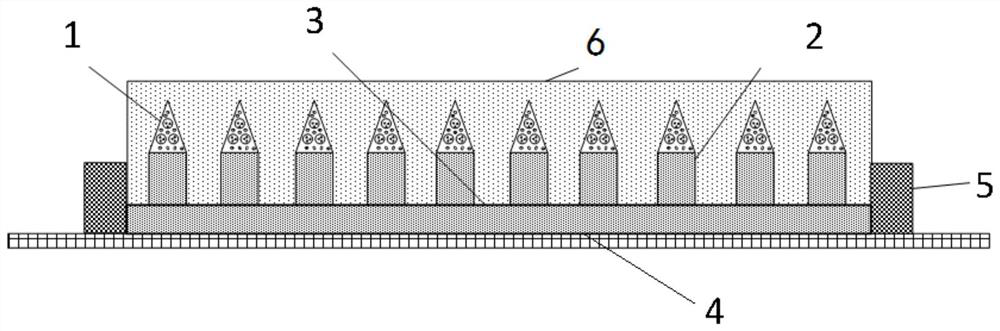
[0028] An embodiment of the microneedle array patch of the present invention, see figure 1 , the microneedle array drug patch described in this embodiment includes a microneedle array and a drug patch, and the microneedle array is placed on the drug patch; the microneedle of the microneedle array includes a soluble drug-loaded microneedle tip 1 and an insoluble microneedle The support end 2; the tip 1 of the soluble drug-loaded microneedle is prepared by mixing BCG, tuberculin microspheres, and soluble excipients through freeze-drying. The microneedle tip 1 is placed on the insoluble microneedle support end 2, the microneedle array base 3 is the insoluble microneedle array base, and the insoluble microneedle support end 2 is located on the surface of the microneedle array base 3; the patch includes a pressure-sensitive adhesive tape 4 and The absorption gasket 5 and the pressure-sensitive adhesive tape 4 are used for pasting the skin and the microneedle array; the hollow part ...
Embodiment 2
[0042] The process of using the microneedle array patch of the present invention is as follows:
[0043] First, remove the sliced medical sponge in the microneedle array patch, and stick the pressure-sensitive tape on the skin, and the whole microneedle array patch is directly pasted on the skin.
[0044] Secondly, press the patch with an appropriate force (no pain is appropriate), stop pressing for 2 minutes, and use the pressure-sensitive tape of the microneedle array patch to fix the depth of the microneedle array piercing into the skin, and the tip of the soluble drug-loaded microneedle Dissolution occurs intradermally.
[0045] Again, tear off the patch after 15 minutes. At this time, the tip of the soluble drug-loaded microneedle completely dissolves and stays in the skin, leaving tiny pores in the stratum corneum of the skin. After about 24 hours, it is completely healed, and the BCG vaccination is completed.
[0046] Finally, in the PPD test, after 3 months, lightly...
Embodiment 3
[0048] Storage stability test of the two-stage microneedle array patch of the present invention
[0049] The two-stage microneedle array patch prepared in Example 1 was placed at room temperature (10-30°C) for 12 months, dissolved in a buffer solution, and the BCG and Mycobacterium tuberculosis contained in the tip of the soluble drug-loaded microneedle were detected Su vitality.
[0050] Among them, BCG was incubated and cultured for 4 weeks, and the number of viable bacteria was detected by a cell counter, and the nucleic acid content of tuberculin was detected by ultraviolet-visible spectrophotometry. The two-stage microneedle array patch just prepared was used as a control to compare the solubility The viable count of BCG contained in the tip of the drug-loaded microneedle and the nucleic acid content of tuberculin.
[0051] The results showed that the number of BCG viable bacteria and the nucleic acid content of tuberculin in the two-stage microneedle array patch that ha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Height | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com