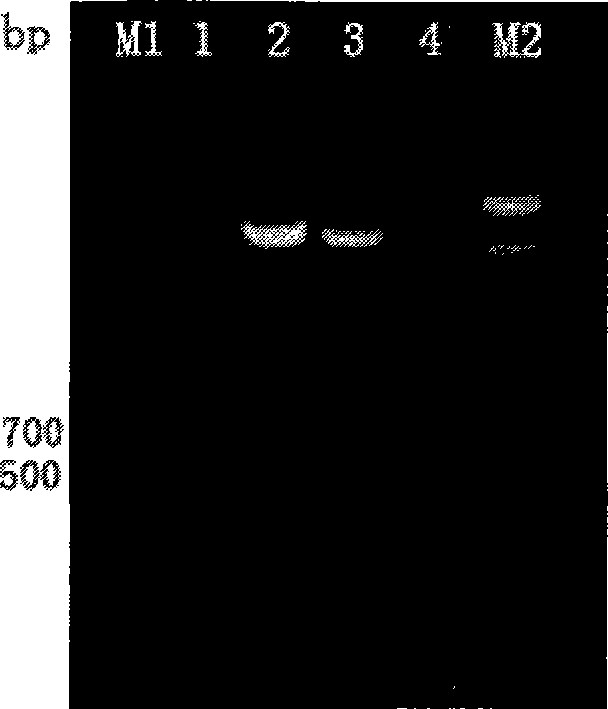
Bacillus coli-mycobacteria shuttling expression plasmid vector and its application in preparation of pathogenic microorganism vaccine
A technology of pathogenic microorganisms and Escherichia coli, applied in the direction of using vectors to introduce foreign genetic material, applications, biochemical equipment and methods, etc., can solve the problems of rising TB morbidity and mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Construction of the Escherichia coli-mycobacterium shuttle vector plasmid pBCG-X suitable for secreting and expressing pathogenic microorganism antigens
[0042] Materials: Escherichia coli-mycobacterium shuttle expression plasmid pBCG-2000, see: Huangpu Yongmu, Cheng Jizhong, etc., Research on the Expression of Foreign Genes in BCG, Journal of Biochemistry, 1997, 13: 370-373.
[0043] 1. Amplification and insertion of Mycobacterium tuberculosis hsp60 promoter into pBCG-2000
[0044] Using the genomic DNA of the standard strain of Mycobacterium tuberculosis H37Rv (GenBank accession number NC_009525) as a template, the primers for PCR amplification of the upstream regulatory sequence of heat shock protein 60 (hsp60) were designed as follows:
[0045] 5' CCGCGG TCGAACGAGGGGCAT3' (the underline is the SacI restriction site)
[0046] 5'TCTAGACATTGCGAAGTGATTCCTCCGGATCG3' (XbaI restriction site is underlined)
[0047] The hsp60 promoter region was amplified by ...
Embodiment 2
[0060] Example 2: Construction of Toxoplasma gondii recombinant BCG-GRA8 vaccine and its immune experiment analysis
[0061] Toxoplasma gondii has a wide range of host sources and numerous isolates. Screening new candidate antigen molecules with strong immunogenicity and specificity, high stability and conservation is the prerequisite for the development of effective diagnostic antigens and practical vaccines. The present invention takes GRA8 as the target antigen, constructs the recombinant BCG vaccine with BCG as the carrier, analyzes its immune response, and observes its protective effect against toxoplasma gondii infection.
[0062] 1. Main experimental materials:
[0063] 1. Animals, cell lines and plasmids 6-8-week-old SPF female BALB / c mice were purchased from the Experimental Animal Center of Sun Yat-sen University School of Medicine; E.coli DH5a was purchased from OMEGA Biotechnology Company, Mycobacterium smegmatis M. smegmatic mc 2 155, from ATCC; pGEM-T carrier, ...
Embodiment 3
[0148] Example 3: Establishment of recombinant Mycobacterium smegmatis vaccine strain expressing SARS virus M protein and its immunogenicity analysis
[0149] This embodiment selects Mycobacterium smegmatis M.smegmatis mc 2 155 as a vaccine vector to construct recombinant Msmc expressing M protein 2 155-M vaccine and analyze its immunogenicity.
[0150] 1. Materials and methods
[0151] 1.1 Materials
[0152] 1.1.1 Animals, cell lines and plasmids
[0153] 6-8-week-old SPF female BALB / c mice were purchased from the Experimental Animal Center of Sun Yat-sen University School of Medicine; E.coli DH5a was purchased from OMEGA Biotechnology Company, Mycobacterium smegmatis M.smegmatic mc 2155, from ATCC; pGEM-T carrier, the product of Promega Company; pMD18-M-TA recombinant plasmid was constructed by the inventor (Lei Mingjun, Wu Shaoting, Dai Wuxing, etc. Cloning and expression of full-length gene of SARS coronavirus M protein and purification. Chinese Journal of Zoonotic Di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com