Adeno-associated virus capsid protein gene, corresponding protein and application of protein
A technology of capsid protein gene and virus, applied in the fields of biomedicine and genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Cloning of the new AAV capsid protein gene of embodiment 1
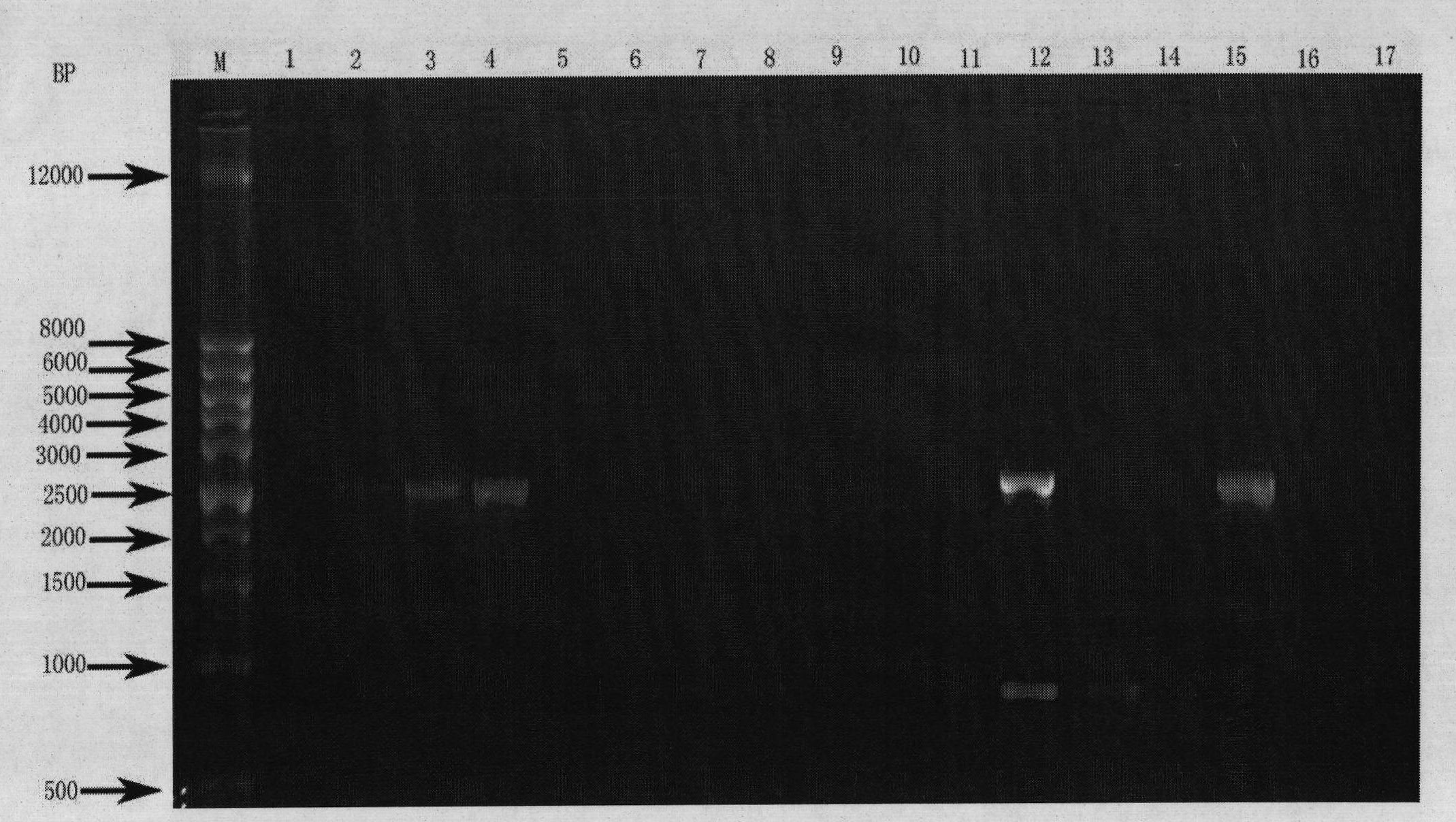
[0027] Human tissue samples were taken, and genomic DNA was extracted with a genomic DNA extraction kit (Qiagen, USA, catalog number: 69504), followed by PCR reaction. The PCR primers used are: the forward primer is shown in SEQ ID NO:3, and the reverse primer is shown in SEQ ID NO:4. PCR primers were synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd. The PCR reaction system is: PCR enzyme 0.25uL (Bao Biological Engineering (Dalian) Co., Ltd., China, product number: DR001A), 10×PCR buffer 5uL, dNTP mixture 4uL (each final concentration is 0.2mM), forward primer (final concentration 0.2uM), reverse primer (final concentration 0.2uM), genomic DNA (100ng), add water to 50uL. The PCR program is: 94 degrees for 30 seconds, 65 degrees for 30 seconds, 72 degrees for 3 minutes, 30 cycles. Take 5uL PCR product for electrophoresis, and the expected band size is about 3.0kb. The result is as ...
Embodiment 2
[0028] Example 2 Preparation of AAV vector using the new AAV capsid protein packaging of the present invention
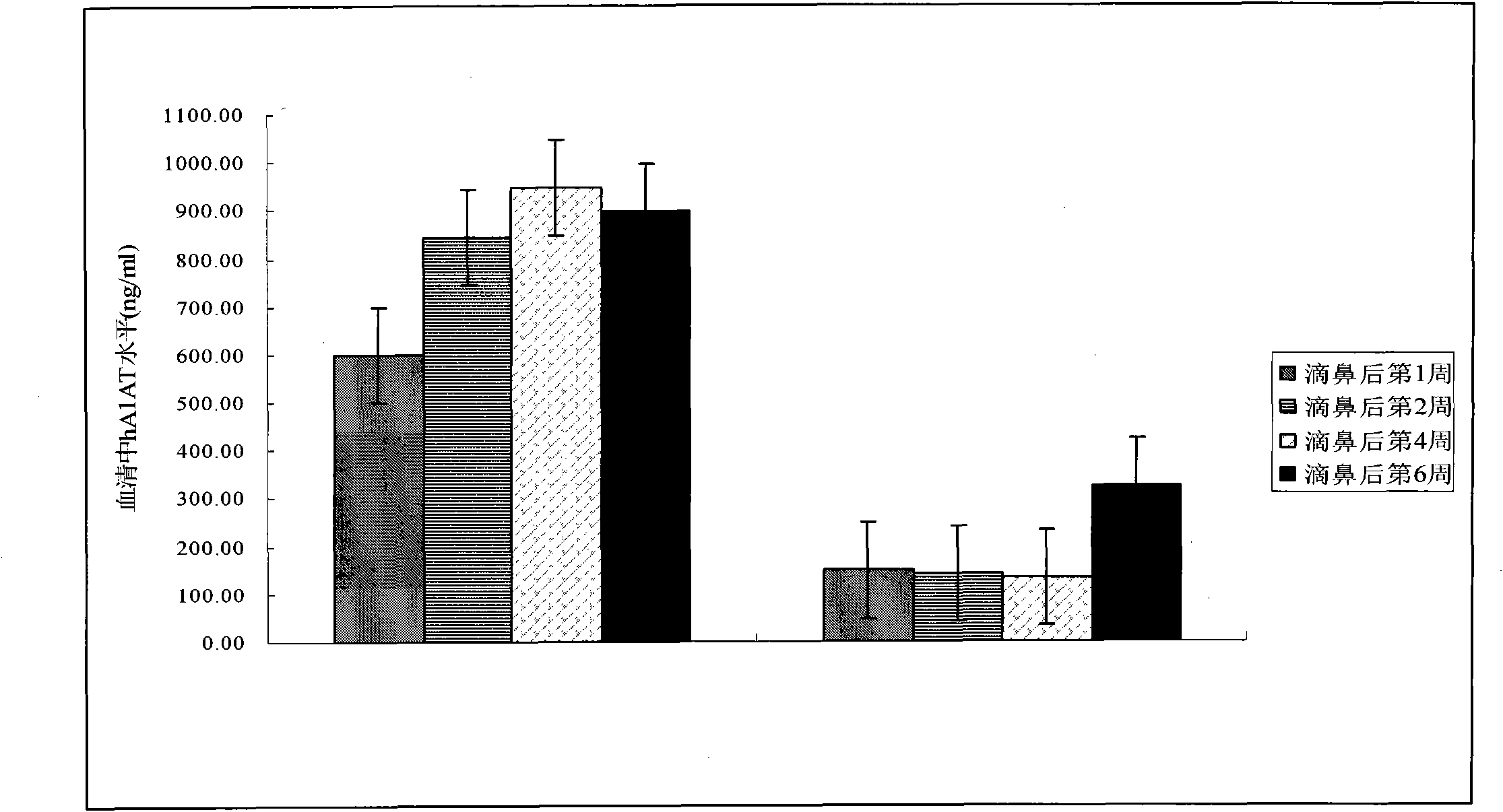
[0029] Preparation of AAV vectors was performed using a triple plasmid co-transfection method. Specifically: take 1000ug of the auxiliary plasmid ΔF6 carrying the adenovirus fragment, 500ug of the cis plasmid pAAV.CMV.EGFP, and 500ug of the plasmid containing the new capsid protein gene, and add CaCl 2 solution to a final concentration of 0.25M, with an equal volume of HBS buffer (5g HEPES, 8g NaCl, 0.37g KCl, 0.14g Na 2 HPO 4 .2H 2 O dissolved in 1 liter of double distilled water) mixed, transfected human embryonic kidney cell line-293 cells. The cells were harvested 3 days after transfection, and after repeated freezing and thawing three times, they were purified according to the literature method (Fisher, Gao, et al., 1996): using cesium chloride isopycnic gradient centrifugation to purify, and adding sterile glycerol after purification (final Concentration: ...
Embodiment 3
[0030] Example 3 In vitro infection experiment of the AAV vector packaged by the new AAV capsid protein of the present invention
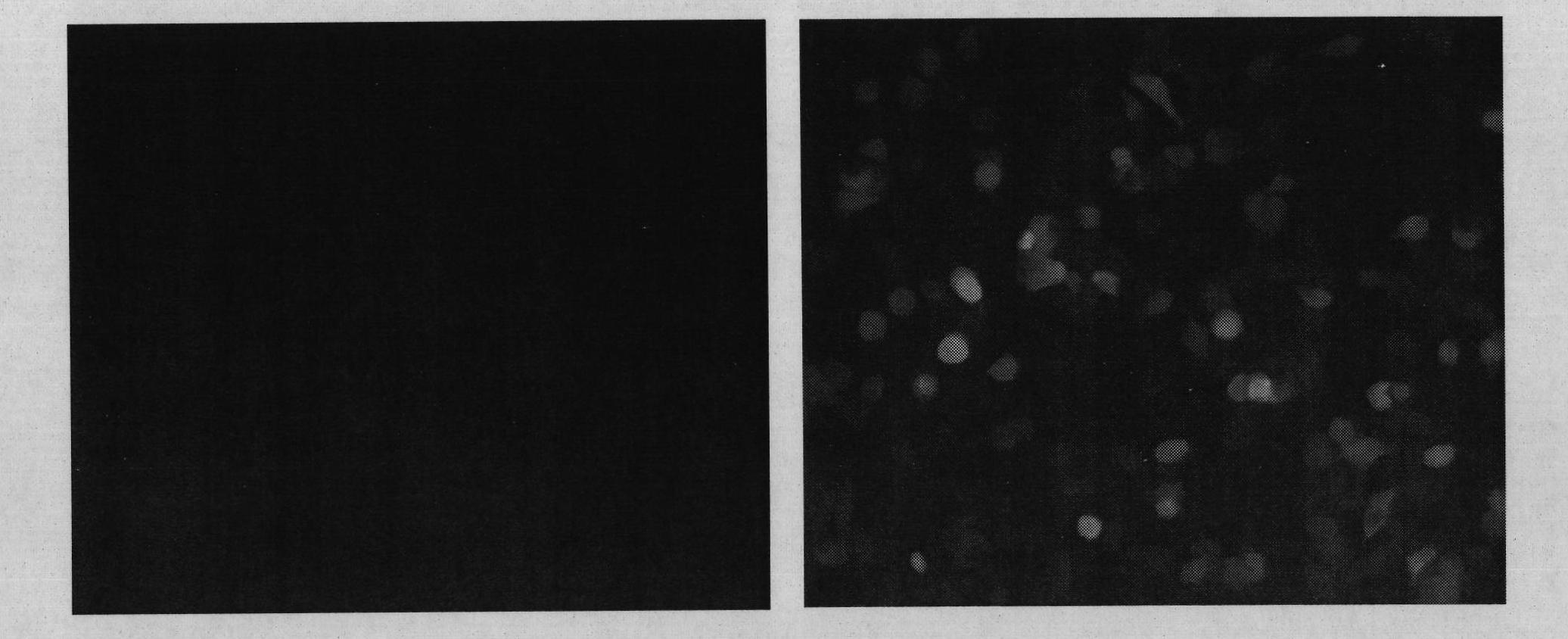
[0031]One day before infection, 293 cells were taken, digested with trypsin / EDTA solution, and plated on a 24-well cell plate at a ratio of 1:2.5. On the day of infection, the culture medium in the cell plate was replaced, and the AAV vector particles prepared according to the above method (Example 2) were added to each well of the cells (1×10 gc of the vector particles per well), and PBS was used as a negative control. Place in cell culture incubator. After culturing for 24 hours, use a fluorescence microscope to observe the expression of green fluorescent protein (EGFP), and take pictures ( figure 2 ). The results show that the AAV vector packaged with the novel AAV capsid protein disclosed by the invention can effectively introduce genes into cells cultured in vitro.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com