Levo-oxiracetam slow-release tablet and preparation method thereof
A technology of slow-release tablets and slow-release matrix materials, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, and medical preparations containing active ingredients, etc. Patient health and other issues, to achieve the effect of reducing adverse drug reactions, reducing the number of times of taking, and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A levoxiracetam slow-release tablet, prepared according to the following steps:
[0026] Chip composition
[0027] Element
Dosage
[0028] Levoxiracetam
1 copy
Hypromellose (K 4 M)
0.65 servings
0.15
Micropowder silica gel
0.06 parts
0.02 parts
0.02 parts
65% ethanol
1.1 copies
[0029] Made into 1000 pieces
[0030] Coating composition:
[0031]
[0032] Preparation process:
[0033] (1) Mix and pulverize levoxiracetam and the slow-release framework material into fine powder (the amount that passes through the No. 5 sieve and the No. 6 sieve that can pass through shall not be less than 95% of the total amount), and sieve;
[0034] (2) Add binder, mix and granulate (pass through 18 mesh sieve), place the prepared wet granules in a hot air oven, set the temperature at 40-60...
Embodiment 2
[0053] A levoxiracetam slow-release tablet, prepared according to the following steps:
[0054] Chip composition
[0055] Element
Dosage
Levoxiracetam
1 copy
Hypromellose (K 4 M)
0.90 servings
0.30 servings
[0056] Micropowder silica gel
0.12 parts
0.05 parts
0.05 parts
65% ethanol
1.5 servings
[0057] Made into 1000 pieces
[0058] Coating composition:
[0059]
[0060] Preparation process: prepared according to the preparation process of Example 1.
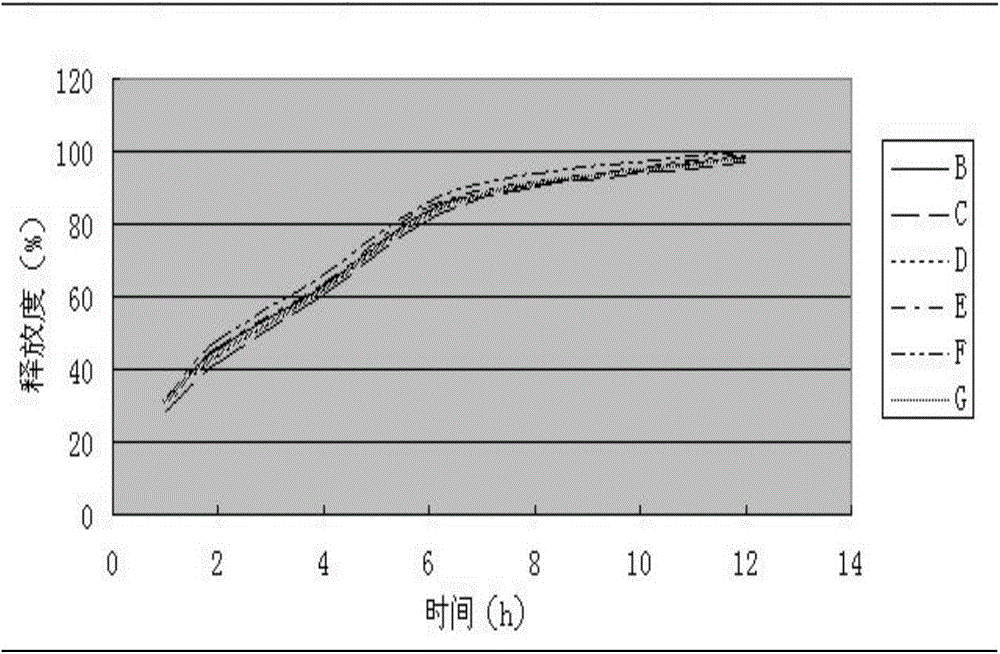
[0061] (1) Determination of release rate
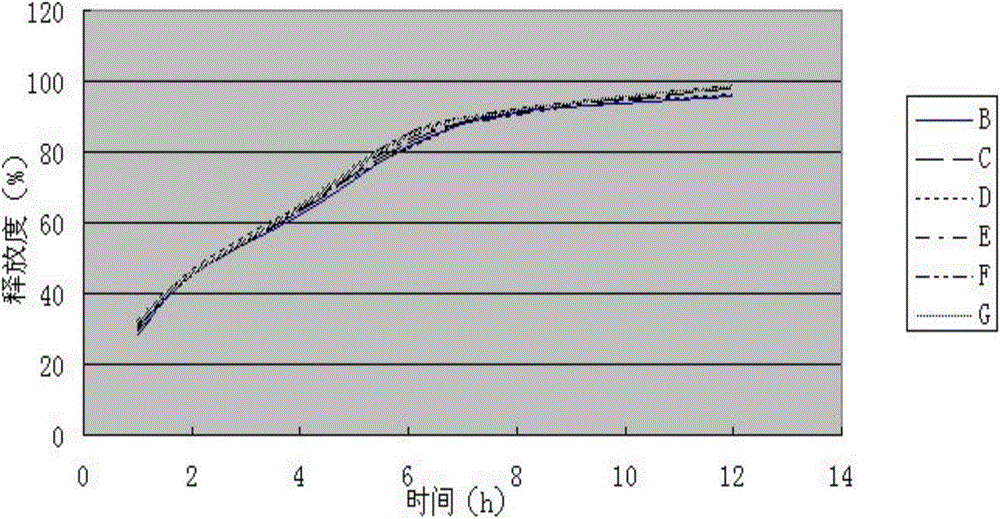
[0062] Measure according to embodiment 1 release degree measuring method, the measurement result of its release degree is shown in Table 2, figure 2 (done six samples to measure).
[0063] Table 2 Sustained-release tablet sample release rate (%) of the present invention
[0064]
[0065...
Embodiment 3
[0069] A levoxiracetam slow-release tablet, prepared according to the following steps:
[0070] Chip composition
[0071] Element
Dosage
Levoxiracetam
1 copy
Hypromellose (K 4 M)
0.85 parts
0.15 parts
Micropowder silica gel
0.12 parts
0.03 copies
0.05 parts
65% ethanol
1.3 parts
[0072] Made into 1000 pieces
[0073] Coating composition:
[0074]
[0075] Preparation process: prepared according to the preparation process of Example 1.
[0076] (1) Determination of release rate
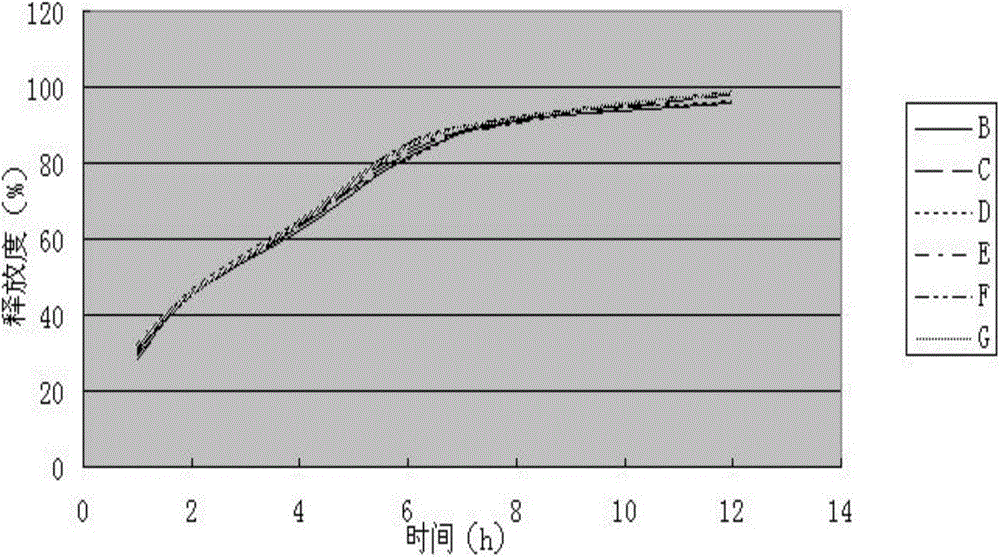
[0077] Measure according to embodiment 1 release degree measuring method, the measurement result of its release degree is shown in Table 3, image 3 (done six samples to measure).
[0078] (1) The assay results of the release of the sustained-release tablet sample of the present invention are shown in Table 3, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com