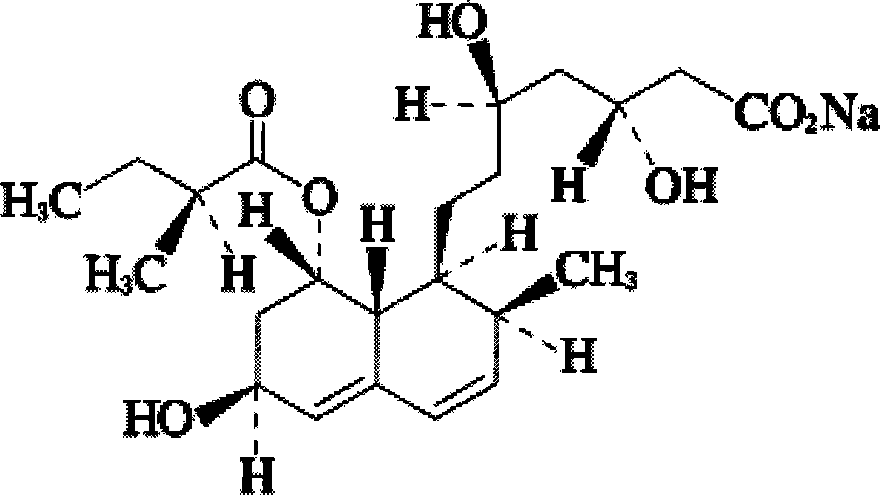
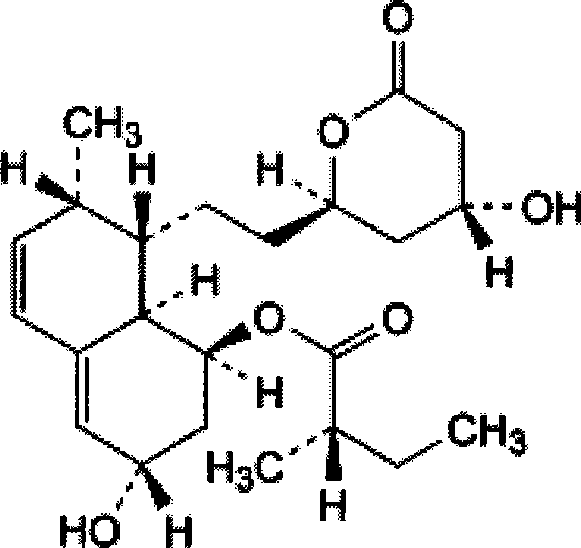
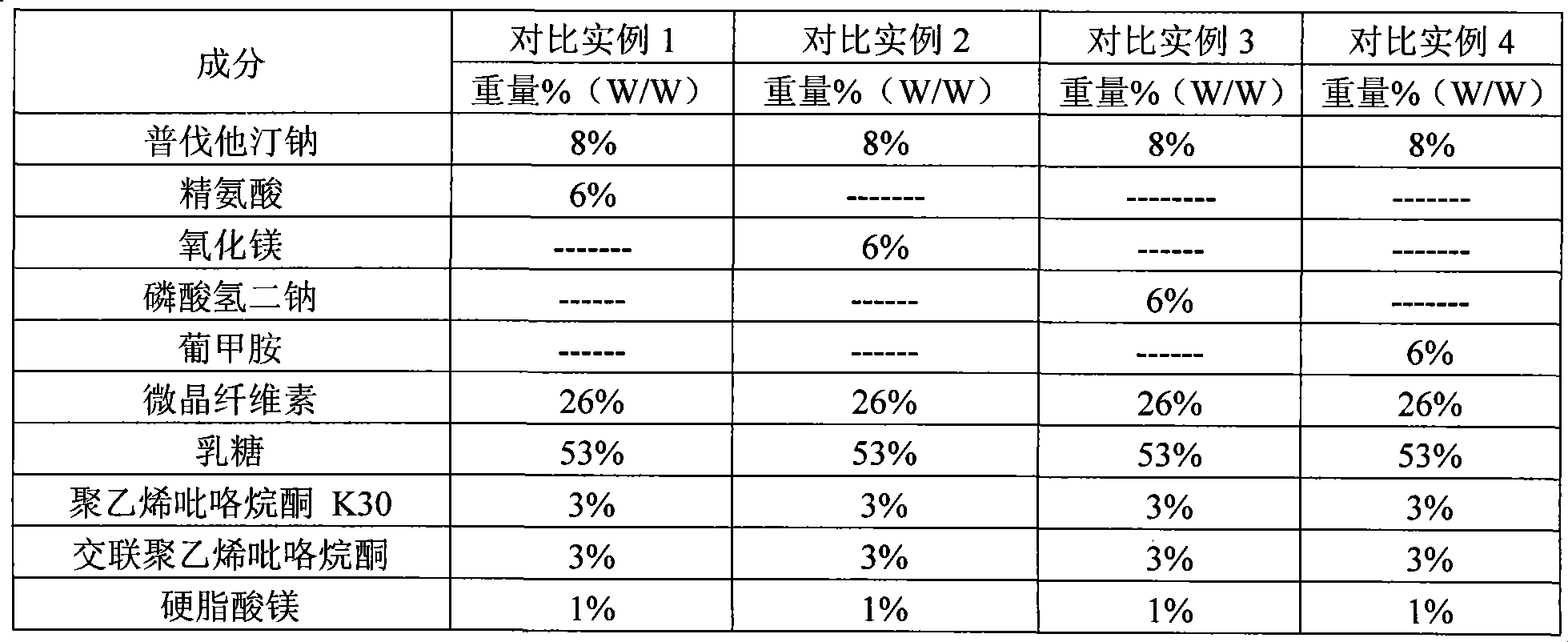
Stable pravastatin medicament composition and preparation method thereof
A technology of pravastatin and composition, applied in the field of pravastatin pharmaceutical composition and its preparation, capable of solving problems such as gastric mucosal injury, gastric mucosal irritation, damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Element Weight % (W / W) Pravastatin Sodium 12% microcrystalline cellulose 48% Croscarmellose Sodium 33% meglumine 5% colloidal silica 0.5% Magnesium stearate 1.5%
[0039] Preparation:
[0040] a Mix pravastatin sodium, microcrystalline cellulose, 2 / 3 croscarmellose sodium, and meglumine;
[0041] b Mix and homogenize the above mixture in a V-type mixer;
[0042] c The above homogenized mixture is granulated in a dry granulator;
[0043] d Mix the remaining croscarmellose sodium, then add colloidal silicon dioxide and magnesium stearate, mix and homogenize;
[0044] e Compress the above mixture into tablets.
[0045] The product of this example is packaged in aluminum and plastic and placed at room temperature for 24 months, the degradation product is only 0.17%, and the appearance does not change color. The results show that the quality of the prepared samples is stable, and the good quality can still be guaranteed ...
Embodiment 2
[0047] Element Weight % (W / W) Pravastatin Sodium 10% microcrystalline cellulose 25% lactose 52% meglumine 6% Polyvinylpyrrolidone K30 3% Cross-linked polyvinylpyrrolidone 3% Magnesium stearate 1%
[0048] Preparation:
[0049] a Mix pravastatin sodium, microcrystalline cellulose, lactose, and meglumine;
[0050] b Mix and homogenize the above mixture in a V-type mixer;
[0051]c Add polyvinylpyrrolidone K30 and cross-linked polyvinylpyrrolidone to the above V-shaped mixer for mixing, then add magnesium stearate to mix and homogenize;
[0052] d Compress the above mixture into tablets.
[0053] The product of this example is packaged in aluminum and plastic and placed at room temperature for 24 months, the degradation product is only 0.13%, and the appearance does not change color. The results show that the quality of the prepared samples is stable, and the good quality can still be guaranteed after long-term storage. ...
Embodiment 3
[0055] Element Weight % (W / W) Pravastatin 20% lactose 60.2% meglumine 20% Croscarmellose Sodium 3% silica 0.8% Magnesium stearate 1%
[0056] The preparation method is the same as in Example 1.
[0057] The product of this example is packaged in aluminum and plastic and placed at room temperature for 24 months, the degradation product is only 0.19%, and no discoloration occurs in appearance. The results show that the quality of the prepared samples is stable, and the good quality can still be guaranteed after long-term storage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com