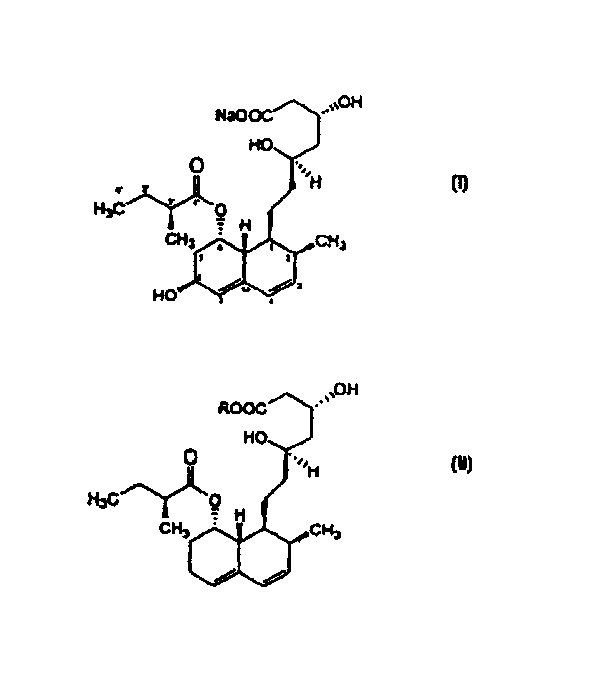
Microbial process for preparing pravastatin
A biotransformation and substrate technology, applied in the direction of microorganism-based methods, methods using fungi, microorganisms, etc., can solve the difficult and difficult problems of DNA technology application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A spore suspension was prepared with 5 ml of 0.9% sodium chloride solution obtained from 7-10 days of Mortierella maculata nov.spec.E-97 [NCAIM(P)F 001266] strain capable of 6β-hydroxylation of compactin Aged wort-yeast extract agar slant cultures were used to inoculate 100 ml of sterilized seed medium PI in a 500 ml Erlenmeyer flask. Composition of medium PI:
[0053] Glucose 50g
[0054] Soy flour 20g
[0055] Dissolved in 1000ml tap water.
[0056] The pH of the medium was adjusted to 7.0 prior to sterilization, followed by sterilization at 121°C for 25 minutes. The culture was shaken on a rotary shaker (250rpm, 2.5cm amplitude) for 3 days at 28°C, and then 10ml of the obtained culture was transferred to 100 ml of the obtained culture in a 500ml Erlenmeyer flask sterilized at 121°C for 25 minutes. -100ml biotransformation medium MU / 4. Composition of medium MU / 4:
[0057] Glucose 40g
[0058] Soy flour 20g
[0059] Casein-Peptone 1g
[0060] Asparagine 2g
...
Embodiment 2
[0066] In a laboratory scale fermentor with a working volume of 5 liters, prepare MU / S biotransformation medium, add medium components corresponding to a volume of 5 liters, but only add up to 4.5 liters, then sterilize at 121°C for 45 minutes, Inoculate with 500 ml of seed culture prepared according to Example 1. Composition of medium MU / 8:
[0067] Glucose 20g
[0068] Glycerin 20g
[0069] Soy flour 20g
[0070] Peptone 5g
[0071] Potassium dihydrogen phosphate 0.5g
[0072] Polypropylene glycol 2000 1g
[0073] Dissolved in 1000ml tap water. The pH of the medium was adjusted to a value of 7.0 prior to sterilization.
[0074] The fermentation was carried out for 4 days at 28°C with a stirring rate of 400 rpm and an aeration rate of 60 liters / hour from the bottom direction. On day 2 after transfection, the culture began to foam heavily, which could be reduced by adding more polypropylene glycol 2000. During the early days of fermentation (16-20 hours), the pH decre...
Embodiment 3
[0078] Biotransformation medium MU / 4 was prepared as described in Example 1 in a laboratory scale 5 liter working volume fermentor, although it loaded up to 4.5 liters, the composition of the medium was calculated at 5 liters. After sterilization at 121°C for 45 minutes, 500 ml of the seed culture prepared according to Example 1 was inoculated. Fermentation was carried out at 25°C for 4 days by using a stirring rate of 300 rpm and an aeration rate of 50 liters / hour. Biotransformation was performed as in Example 2 after adding 5 g of compactin substrate to the culture.
[0079] After completing this generative transformation, 4.9 liters of broth containing 660 μg / ml pravastatin were filtered and the isolated mycelia washed by suspending in 2 x 1 liter deionized water. The pH of the combined 5.6 liter broth filtrate was adjusted to 3.5-3.7 with 20% sulfuric acid, and the acidic filtrate was stirred with 2750 ml of ethyl acetate for 30 minutes. Subsequently, the phases are sepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com