Manufacturing method of atorvastatin intermediate (R)-(-)-4-nitrile-3-hydroxybutyrate
A technology of ethyl hydroxybutyrate and atorvastatin, applied in the fields of medicine and chemical industry, can solve the problems of unsatisfactory purity of intermediate I, low stereoselectivity, expensive catalyst, etc., and achieves short reaction process and abundant raw material sources. , the effect of low price of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
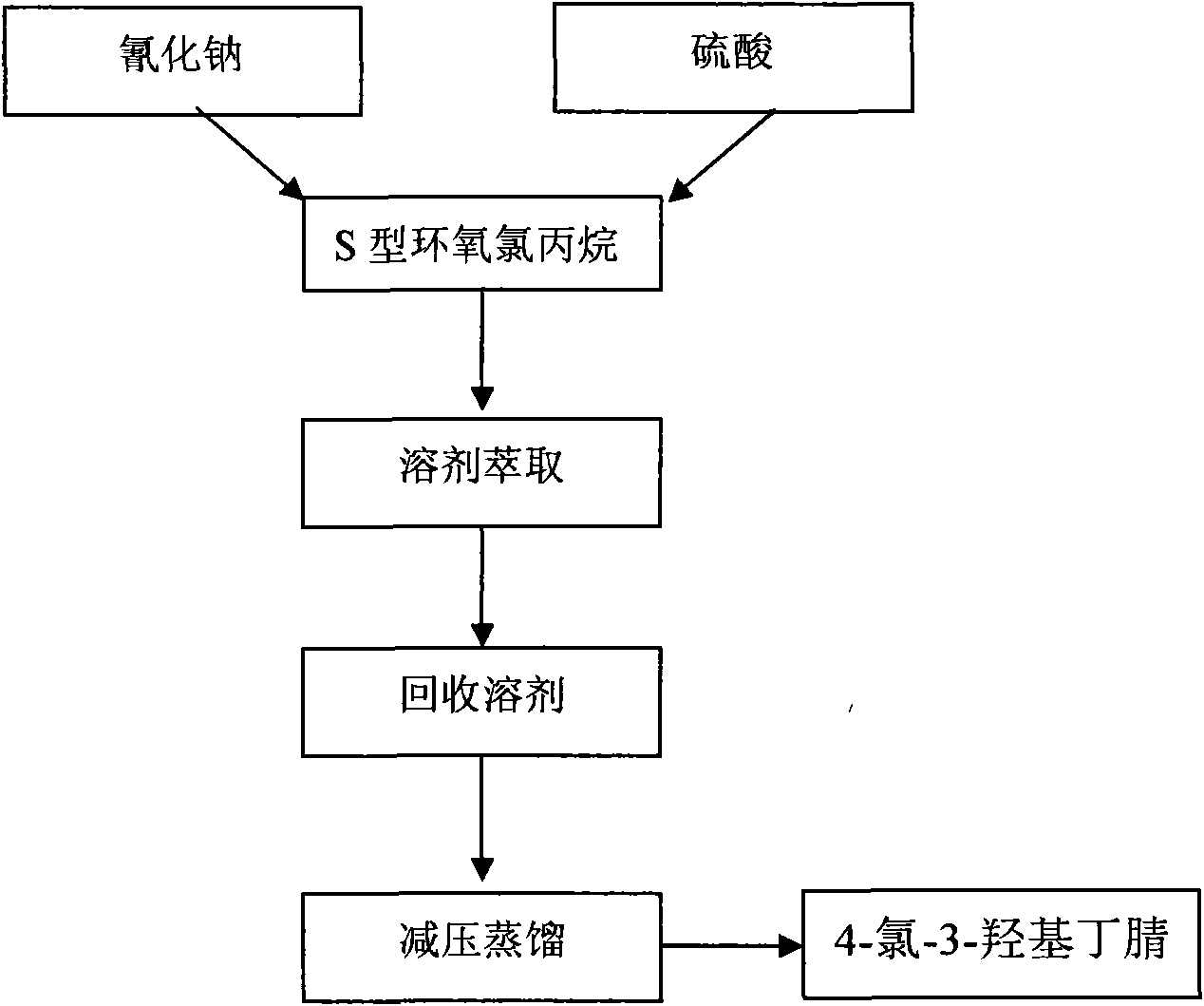
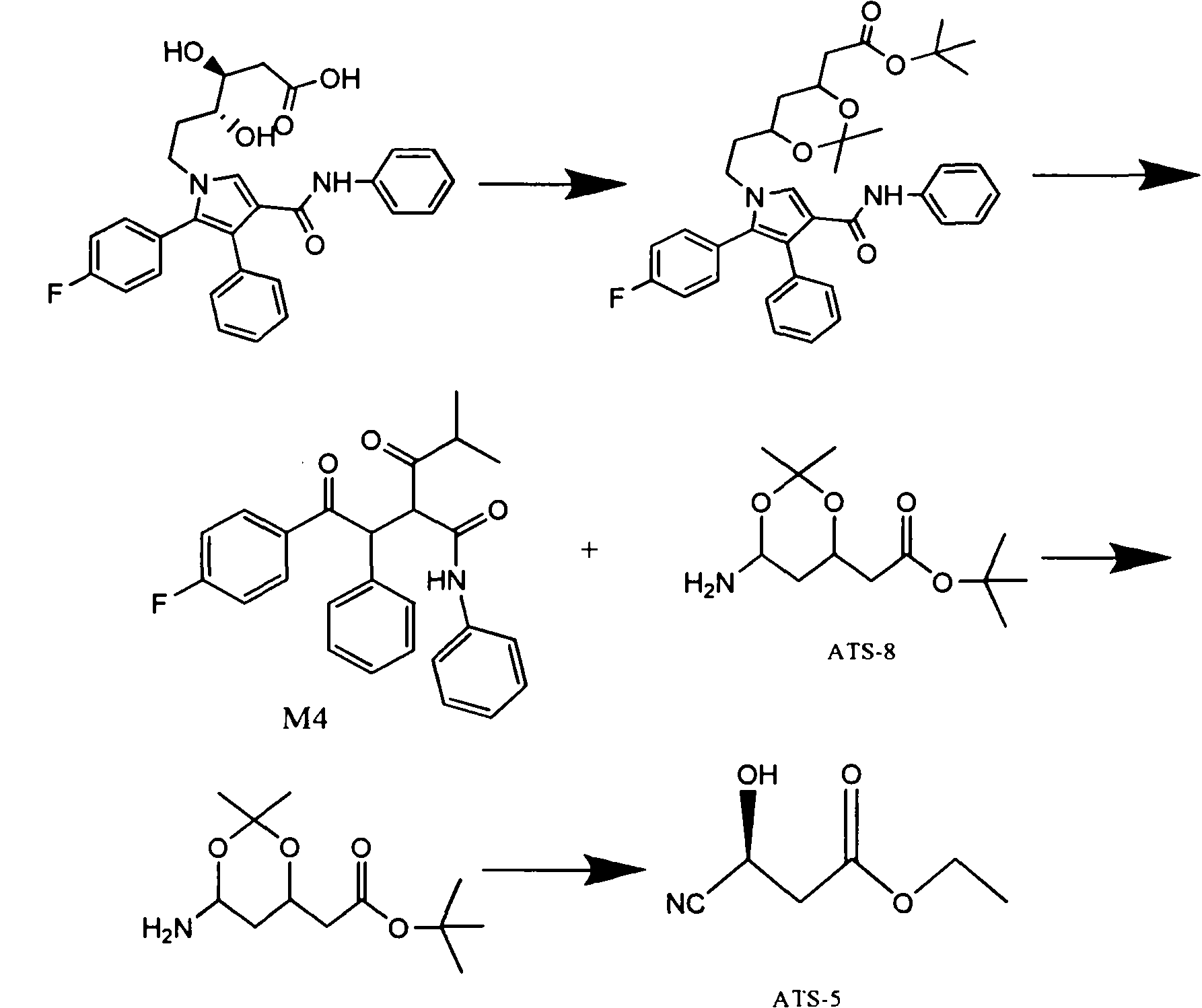
[0038] Take the industrialized production of ATS-5 with an annual output of 40 tons as an example:
[0039] The first process is: split epichlorohydrin to obtain S-epichlorohydrin. The R configuration or S configuration of epichlorohydrin reacts with the resolving agent (chiral reagent) B to form complexes R.B or S.B respectively. There is no mirror image relationship between them, and the energy of the two is also different; therefore, the R type When Type S and Type B react with B respectively, their activation energies are different, and their reaction speeds are also different. The relative speed of hydrolysis of S-type and R-type epichlorohydrin is different, and the hydrolysis speed of R-type epichlorohydrin is faster than that of S-type epichlorohydrin, and the hydrolysis speed of epichlorohydrin is proportional to the square of concentration (is a two level reaction kinetics). After a period of reaction, the R-type epichlorohydrin is completely hydrolyzed, and a smal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com