Pharmaceutical compositions of amlodipine and atorvastatin
A technology of atorvastatin and atorvastatin calcium, which is applied in the field of pharmaceutical compositions of amlodipine and atorvastatin, and can solve problems such as inability to prove complete normalization of cardiovascular mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] General Process for Manufacture of Atorvastatin Calcium / Amlodipine Besylate Dual Therapy Tablets
[0217] [A] Atorvastatin granulation method
[0218] Step 1. - Dissolve polysorbate 80 in purified water at 50°C and add hydrated hydroxypropylcellulose. The solution was allowed to cool to room temperature.
[0219] Step 2.- Mix atorvastatin calcium, calcium carbonate, microcrystalline cellulose, starch 1500 and croscarmellose sodium in a fluid bed granulator / dryer (FBG / D) or high shear mixer / granulator .
[0220] Step 3.- Granulate the powder mixture from step 2 and the solution from step 1 in a FBG / D or high shear mixer / granulator.
[0221] Step 4.- Dry the granules in FBG / D or other drying equipment to a moisture content (loss on drying, LOD) less than or equal to 2.0%.
[0222] [B] final preparation
[0223] Step 1.- Add amlodipine besylate, microcrystalline cellulose, croscarmellose sodium and silicon dioxide to the atorvastatin granules obtained in step [A].
...
Embodiment 2
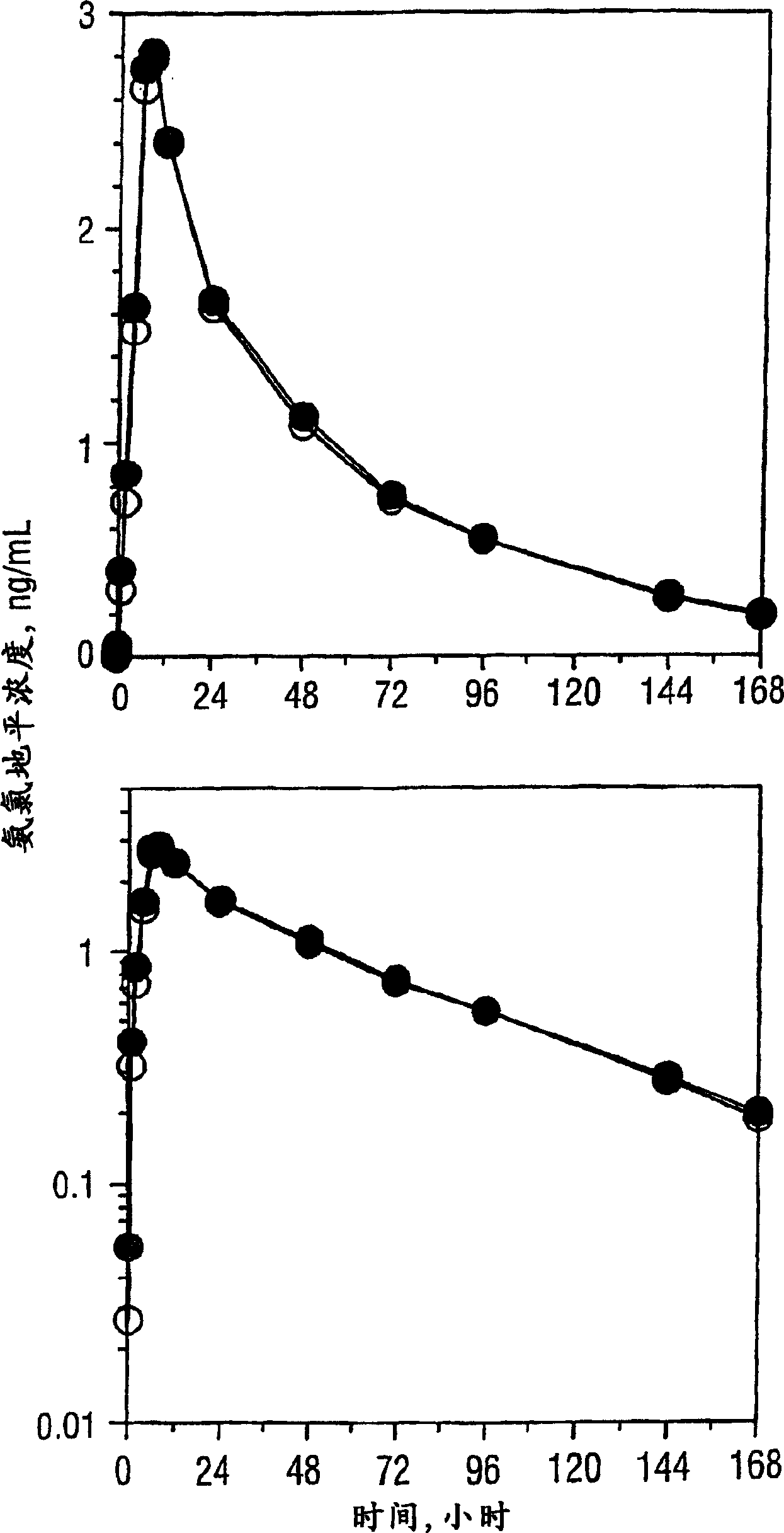
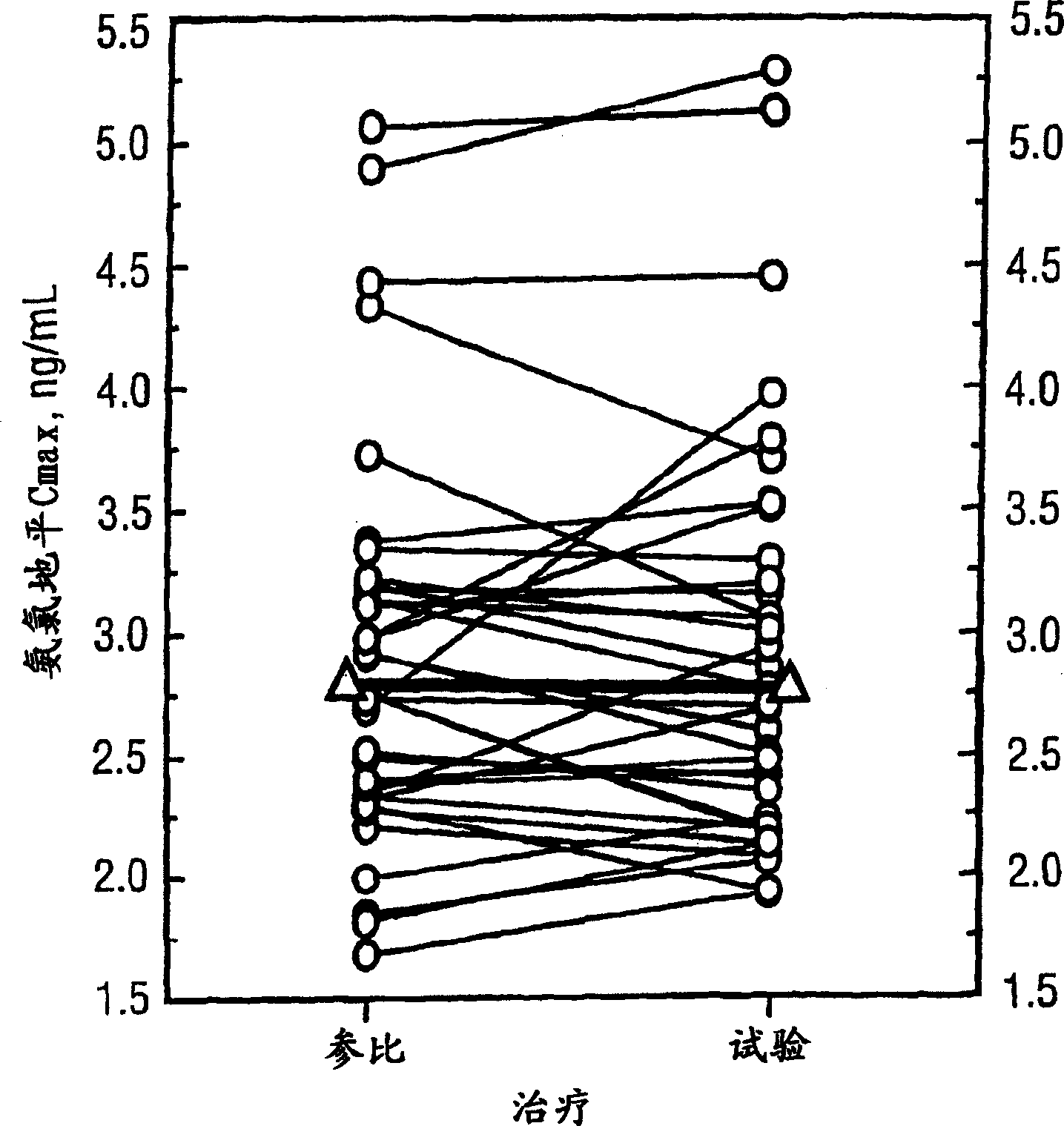
[0251] A Single-Dose Bioequivalence Study of 5-mg Amlodipine / 10-mg Atorvastatin Dual Therapy Tablets Compared with Coadministered 5-mg Amlodipine and 10-mg Atorvastatin Tablets
[0252] Protocol: A randomized single-dose two-way crossover study in 36 healthy volunteers. Following an overnight fast, each subject received a single 5-mg amlodipine and 10-mg atorvastatin tablet as a dual treatment and co-administered separate tablets on Days 1 and 15.
[0253] Blood samples were collected before and for 168 consecutive hours after each dose. Plasma samples were collected and stored frozen at -70°C until analysis. Plasma amlodipine and atorvastatin concentrations were determined by validated methods. Pharmacokinetic parameter values were evaluated from concentration-time curves by non-compartmental methods. ANOVA (Analysis of Variance) results of the log-transformed Cmax and AUC values were used to calculate 90% confidence intervals for the proportion of means with the least...
Embodiment 3
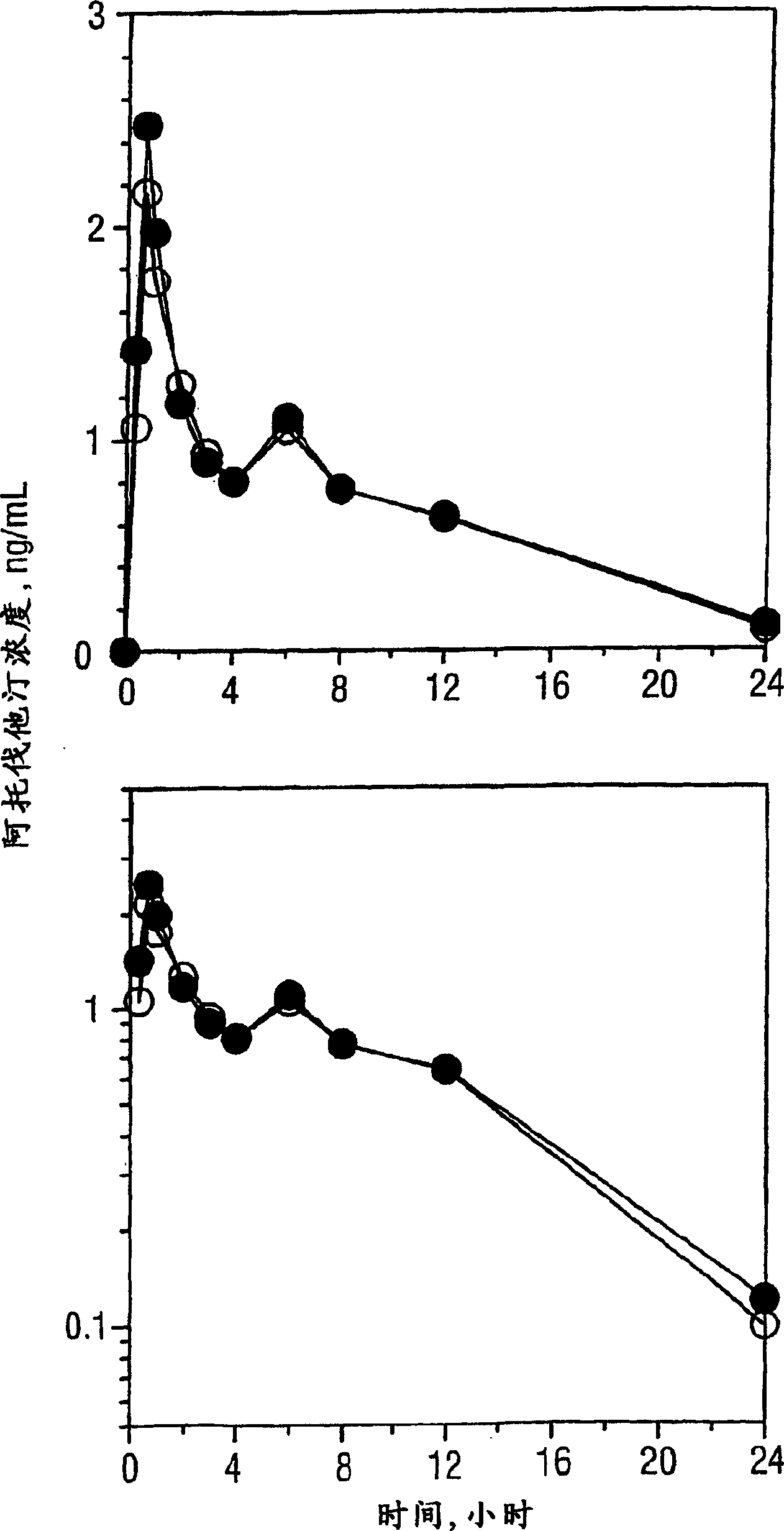
[0269] A Single-Dose Bioequivalence Study of 10-mg Amlodipine / 40-mg Atorvastatin Dual Therapy Tablets Compared with Coadministered 10-mg Amlodipine and 40-mg Atorvastatin Tablets
[0270] Protocol: A randomized single-dose two-way crossover study in 36 healthy volunteers. Following an overnight fast, each subject received a single 10-mg amlodipine and 40-mg atorvastatin as a dual treatment tablet and co-administered separate tablets on Days 1 and 15.
[0271] Blood samples were collected before and for 168 consecutive hours after each dose. Plasma samples were collected and stored frozen at -70°C until analysis. Plasma amlodipine and atorvastatin concentrations were determined by validated methods. Pharmacokinetic parameter values were evaluated from concentration-time curves by non-compartmental methods. ANOVA results of log-transformed Cmax and AUC values were used to calculate 90% confidence intervals for the proportion of mean values of the least variance treatmen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com