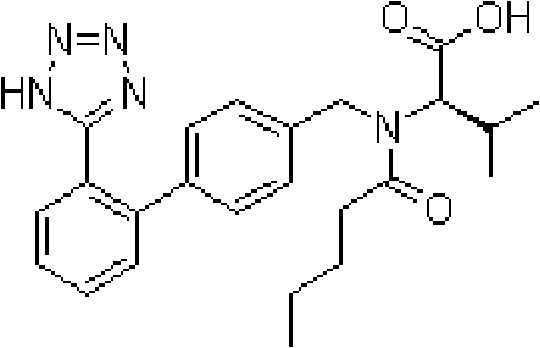
A kind of valsartan capsule and preparation method thereof
A valsartan and capsule technology, applied in the field of medicine, can solve the problems of unfavorable dissolution stability of valsartan preparation products, inconvenience for patients to take, slow dissolution speed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] prescription:
[0092]
[0093] Preparation:
[0094] (1) Adhesive preparation: Weigh the prescription amount of sodium lauryl sulfate and povidone K30, dissolve in water, and prepare a 10% povidone K30 solution;
[0095] (2) Sieving: the auxiliary materials of microcrystalline cellulose, crospovidone and magnesium stearate are passed through a 60-mesh sieve;
[0096] (3) Granulation and drying: Weigh the prescription amount of valsartan raw materials into a mixer, add the above-mentioned binder to make the soft material, and then granulate with a granulating machine; the wet granules are dried at 40℃~45℃ for 1~ After 3 hours, whole grain;
[0097] (4) Mixing: Put the above-mentioned granules in a mixer, add the prescribed amount of microcrystalline cellulose, crospovidone and magnesium stearate, mix for 30 minutes, take samples to determine the content and loss on drying;
[0098] (5) Filling: Calculate the average filling volume according to the results of the particle content ...
Embodiment 11
[0104] The pretreatment method of Example 11 is:
[0105] i) Dissolve 12g of valsartan in 100ml of ethanol to obtain a 0.12g / ml valsartan ethanol solution;
[0106] ii) Dissolve 5g of Povidone K30 in 100ml of water to prepare an aqueous solution of Povidone K30 with a concentration of 5%;
[0107] iii) Put the povidone K30 aqueous solution of step ii) in an ice water bath at 0-5°C, and add the valsartan ethanol solution dropwise at a rate of 1.2ml / min under the condition of ultrasound at a frequency of 0.4KW In the povidone K30 aqueous solution, until the solution system becomes turbid, let it stand at 0-5°C for 7 hours, filter, dry, and pulverize through an 80 mesh sieve.
Embodiment 12
[0108] The pretreatment method of Example 12 is:
[0109] i) Dissolve valsartan in ethanol to obtain an ethanol solution of valsartan;
[0110] ii) Dissolve povidone K30 in water to prepare an aqueous solution of povidone K30;
[0111] iii) Put the povidone K30 aqueous solution of step ii) in an ice water bath, add the valsartan ethanol solution dropwise to the povidone K30 aqueous solution at a uniform rate under ultrasonic conditions, until the solution system becomes turbid, stand still, filter, Dry, crush and sieve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com