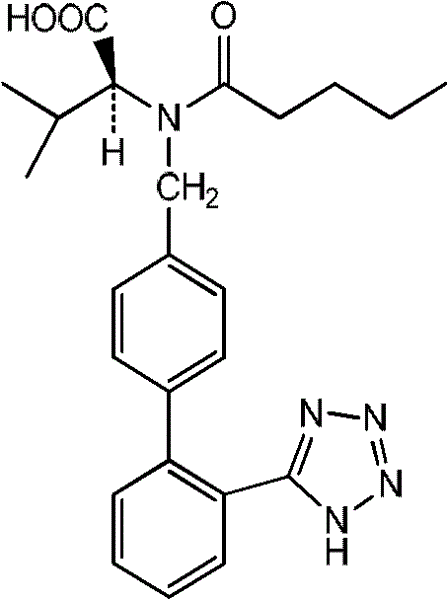
Process for refining valsartan
A purification method and valsartan technology are applied in the field of industrial purification of valsartan, can solve the problems of no industrial purification method of valsartan, few literature reports, low purification yield and the like, and achieve the advantages of being environmentally friendly and easy to operate. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 valsartan crude product
[0026] Crude valsartan can be prepared by the existing preparation method, such as the existing 4-bromomethyl-2'-cyanobiphenyl as raw material and L-valine methyl ester condensation, and then valerylation, cyclization and the crude valsartan prepared by hydrolysis (see: US Patent No. 7,741,507), the quality of the crude valsartan: chromatographic purity 91%, chiral isomer: 4%.
Embodiment 2
[0027] The refining of embodiment 2 valsartan crude product
[0028] Add 100kg of crude valsartan in Example 1 (loss on drying: 20%) to a 500L reactor, then add 160kg of ethanol, stir and dissolve at 20°C, after dissolving, add 40kg of calcium hydroxide, and stir at 20°C for 2 hours , filter to get the calcium salt of valsartan, the calcium salt of valsartan is added in the reactor of 1000L, add 450kg ethyl acetate, then add the dilute hydrochloric acid aqueous solution that mass percent concentration is 9% to adjust pH=2, After the adjustment, separate layers, wash the ester solvent layer twice, evaporate to dryness under reduced pressure to recover the dry solvent, add 432kg ethyl acetate to dissolve, after dissolving, transfer to the special kettle for crystallization of valsartan (disclosed in Chinese patent ZL201020216357.0 A special kettle for valsartan crystallization) was stirred, slowly cooled to -2.5°C ± 2.5°C, centrifuged, and dried under reduced pressure to obtain ...
Embodiment 3
[0029] The refining of embodiment 3 valsartan crude product
[0030] Add 100kg of crude valsartan in Example 1 (loss on drying: 20%) to a 1000L reactor, then add 500kg of ethyl acetate, stir and dissolve at 30°C, after dissolving, add 40kg of calcium hydroxide, stir at 30°C After 2 hours, filter to obtain the calcium salt of valsartan, add the calcium salt of valsartan in a 1000L reaction kettle, add 450kg of ethyl acetate, then add a 9% dilute hydrochloric acid aqueous solution to adjust the pH= 2. After adjustment, separate layers, wash the ester solvent layer with water twice, evaporate to dryness under reduced pressure to recover the dry solvent, add 432kg of ethyl acetate to dissolve, after dissolving, transfer to the special kettle for valsartan crystallization (Chinese patent ZL201020216357.0 A disclosed special kettle for valsartan crystallization) was stirred, slowly cooled to -2.5°C ± 2.5°C, centrifuged, and dried under reduced pressure to obtain 68kg of valsartan, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com