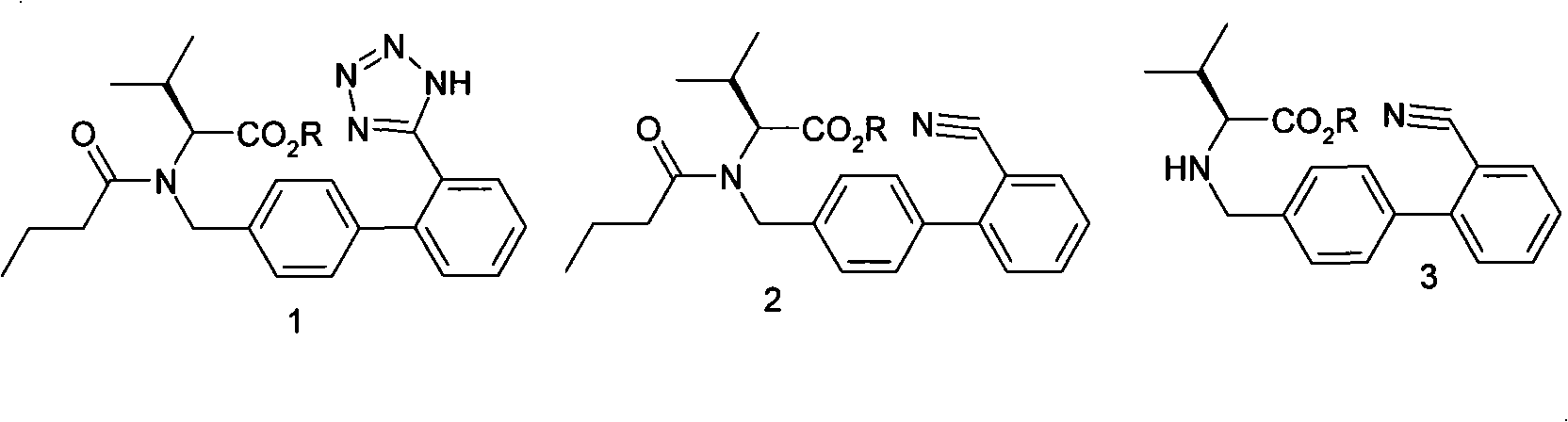
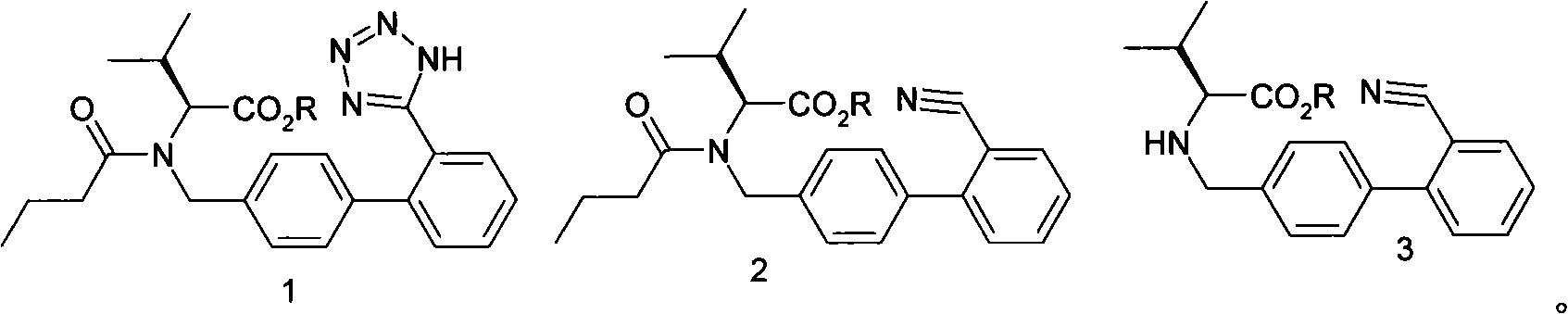
Method for synthesizing diovan
A technology of valsartan and compound is applied in the field of synthesizing valsartan, and achieves the effects of high yield, low price, and avoiding excessive heavy metal tin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0015] Add 50L of drinking water, then add 25kg of potassium carbonate and stir until it is dissolved, then add 20kg of condensate hydrochloride and 150L of toluene, and stir until it is dissolved. Control the temperature at 20-30°C and start adding dropwise the mixture of 9kg valeryl chloride and 25L toluene, the dropwise addition is completed in about 2 hours, then keep the temperature at 20-30°C and stir the reaction for 1 hour, let stand to separate and separate the lower water layer, The organic layer was washed with saturated brine. The saline layer was separated, and the organic layer (pentanoylate solution) was set aside. Use the pentanoyl compound solution prepared in the previous step, add 7.3Kg of sodium azide and 17.8kg of triethylamine hydrochloride, stir evenly, heat to reflux, react for 20 hours, cool to 30°C after reflux, and then add saturated saline 50L, let it stand for stratification. The brine layer was removed, and the organic layer was washed with an a...
example 2
[0017] Except replacing the salt of wormwood in the example 1 with sodium carbonate, all the other are the same as the example 1, and the yield is 75%.
example 3
[0019] Except replacing the potassium carbonate in example 1 with sodium bicarbonate, all the other are with example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com