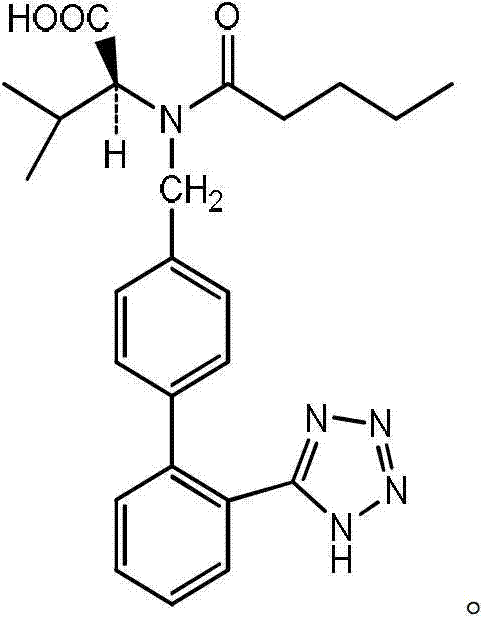
Method for crystallizing valsartan
A technology of valsartan and crystallization, applied in the crystallization field of valsartan, can solve the problems of high cost, few literature reports, low yield and the like, and achieves the effects of simple operation, simple operation and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 valsartan crude product
[0033] Crude valsartan can be prepared by existing preparation methods, such as the existing 4-bromomethyl-2'-cyanobiphenyl as raw material and L-valine methyl ester condensation, followed by valerylation and cyclization and hydrolysis to prepare crude valsartan (see: US Patent US7741507), quality: chromatographic purity 93%, chiral isomers: 3.7%, loss on drying: 20%.
Embodiment 2
[0034] The refining of embodiment 2 valsartan crude product
[0035] Add 400kg of crude valsartan to a 5000L reactor, then add 2000kg of ethyl acetate, stir and dissolve, wash twice with 200kg of drinking water, after washing, recover ethyl acetate from the ethyl acetate layer until dry, then add 1920kg Ethyl acetate, heated up to 40°C to dissolve, after dissolving, add 640kg cyclohexane, after adding, stir and cool down to 18°C, keep warm for 12 hours, then slowly cool down to 0°C at a cooling rate of 0.1°C / min, keep warm After 4 hours, centrifuge and dry to obtain 290kg of valsartan, with a total refined yield of 90.6%, quality: chromatographic purity of 99.88%, chiral isomers of 0.25%, and a single residual solvent of less than 100ppm.
Embodiment 3
[0036] The refining of embodiment 3 valsartan crude product
[0037] Add 400kg of crude valsartan to a 5000L reactor, then add 2000kg of ethyl acetate, stir and dissolve, wash twice with 200kg of drinking water, after washing, recover ethyl acetate from the ethyl acetate layer until dry, then add 2240kg Ethyl acetate, heated up to 40°C to dissolve, after dissolving, add 960kg cyclohexane, after adding, stir and cool down to 18°C, keep warm for 12 hours, then slowly cool down to 0°C at a cooling rate of 0.5°C / min, keep warm After 4 hours, centrifuge and dry to obtain 280kg of valsartan, with a total refined yield of 87.5%, quality: chromatographic purity of 99.78%, chiral isomers of 0.34%, and a single residual solvent of less than 100ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com