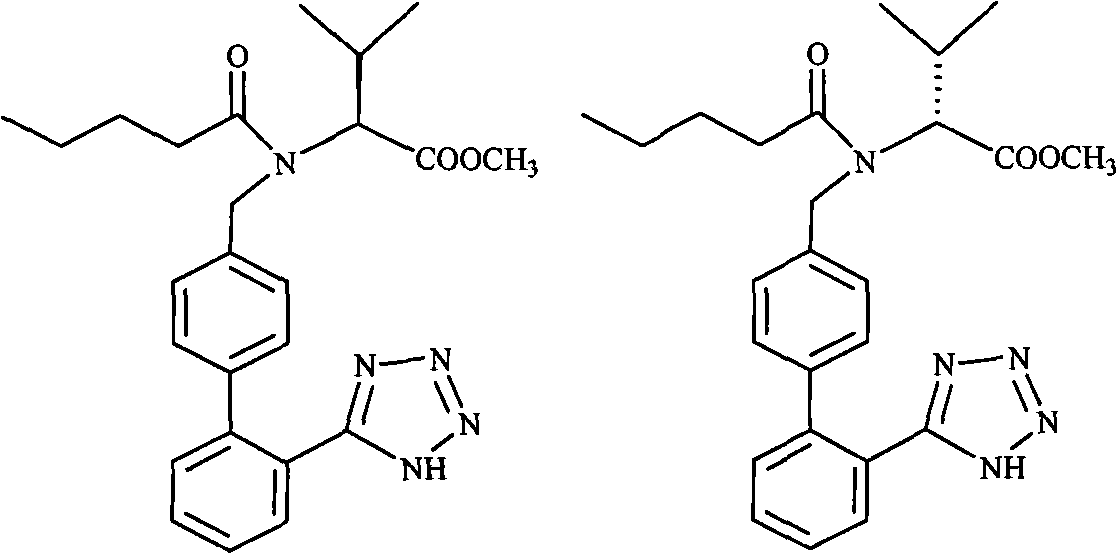
Method for refining Valsartan containing more than 10% of isomer
A purification method and isomer technology, applied in the field of medicine and chemical industry, can solve problems such as cost increase, no reported purification method, etc., and achieve the effect of reducing D-type isomer and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 20.0g of valsartan containing 10.2% of the D-isomer (HPLC detection peak area method) and 60ml of butanone into a 100ml three-necked flask, heat to 40°C and stir to dissolve, then cool to -15°C and stir to crystallize, and precipitate Stirring was continued for about 5 hours, suction filtered, natural drying in a fume hood, and then vacuum drying at 30° C. to obtain 7.9 g of solid with a yield of 39%. The D-isomer was detected by HPLC as 0.28%.
Embodiment 2
[0018] Add 20.0g of valsartan containing 12.9% of the D-isomer (HPLC detection peak area method) and 100ml of butanone into a 250ml three-necked flask, heat to 40°C and stir to dissolve, then cool to -15°C and stir to crystallize, and precipitate Stirring was continued for about 5 hours, suction filtered, natural drying in a fume hood, and then vacuum drying at 30° C. to obtain 3.2 g of solid with a yield of 16%. The D-isomer detected by HPLC was 1.42%.
Embodiment 3
[0020] Add 25.0g of valsartan containing 12.9% of D-isomer (HPLC detection peak area method) and 125ml of butanone in a 500ml three-necked flask, heat to 40°C and stir to dissolve, then add 140ml of isopropyl ether, and cool to -15 Stir and crystallize at ℃, continue to stir for about 5 hours, filter with suction, and dry in vacuo at 35°C to obtain 12.7 g of solid with a yield of 50.8%. The D-isomer detected by HPLC is 3.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com