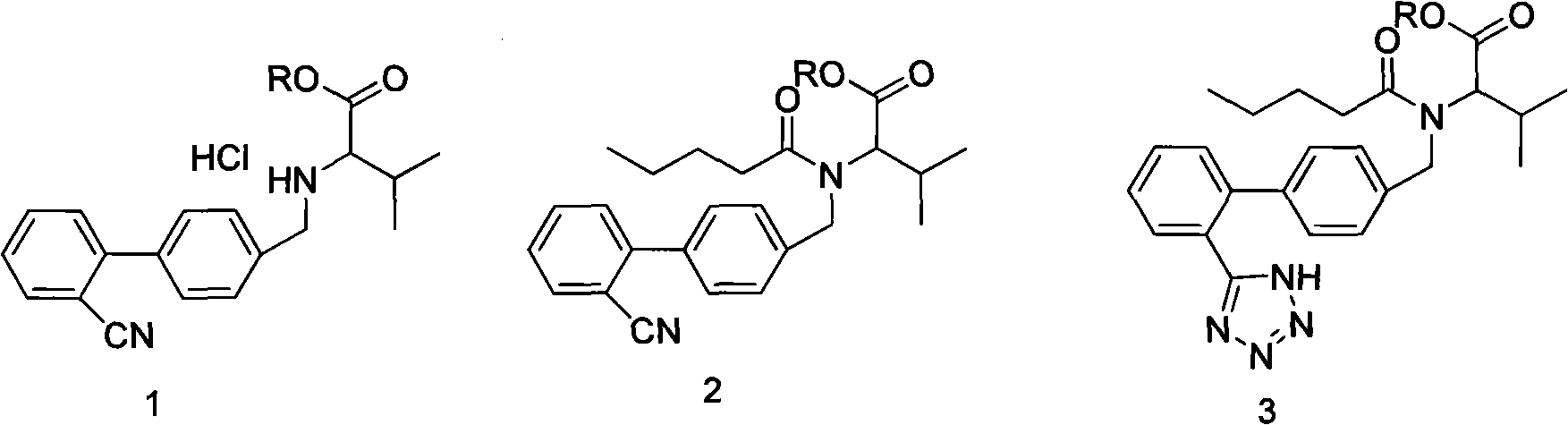
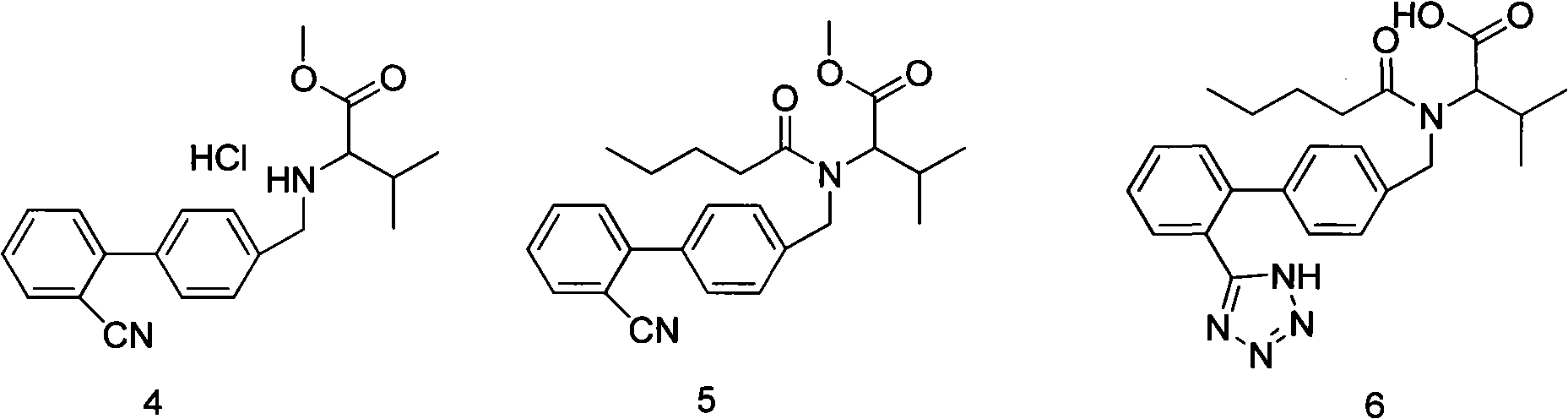
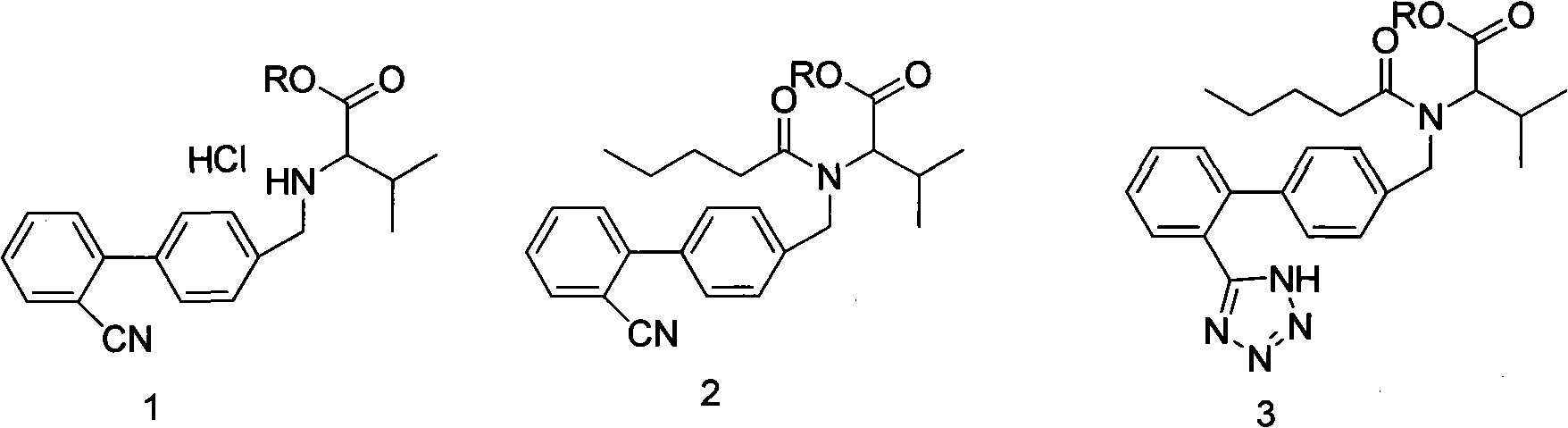
Improved method for synthesizing valsartan
A compound, the technology of valine alkyl ester, applied in the direction of organic chemistry, can solve the problems of low yield, difficult to reduce the content of optical isomers, difficult to control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Example 1: Synthesis of Valsartan
[0026] Add 200ml of water, 25g of sodium carbonate, 350ml of toluene and 28g of condensate hydrochloride into the reaction bottle, stir to dissolve, cool down to 10°C, add 18g of valeryl chloride dropwise, and keep it warm at 10°C for 2 hours. After the reaction is complete, let it stand The layers were separated, and the lower aqueous layer was separated. The organic layer was washed with sodium bicarbonate solution and dilute hydrochloric acid, dried over magnesium sulfate, and filtered to obtain a pentanoylation reaction solution. Add 11 g of sodium azide and 23.5 g of triethylamine hydrochloride to the valerylation reaction solution, and react under reflux for 30 hours, cool down to 50°C, add 200ml of 8% sodium hydroxide solution, and react at 25°C for 4 hours, statically Separate the layers, separate the organic layer, use 6M dilute hydrochloric acid to adjust the pH of the reaction solution to 1-2, add 200ml of isopropyl acetate...
example 2
[0027] Example 2: Synthesis of Valsartan
[0028] Add 200ml of water, 25g of sodium carbonate, 350ml of toluene and 28g of condensate hydrochloride into the reaction bottle, stir to dissolve, cool down to 10°C, add 18g of valeryl chloride dropwise, and keep it warm at 10°C for 2 hours. After the reaction is complete, let it stand The layers were separated, and the lower aqueous layer was separated. The organic layer was washed with sodium bicarbonate solution and dilute hydrochloric acid, dried over magnesium sulfate, and filtered to obtain a pentanoylation reaction solution. Add 11g of sodium azide and 23.5g of triethylamine hydrochloride to the valerylation reaction solution, reflux for 25 hours, cool down to 50°C, add 200ml
[0029] 8% sodium hydroxide solution, reacted at 20°C for 4 hours, let stand to separate layers, separated the organic layer, adjusted the pH of the reaction solution to 1-2 with 6M dilute hydrochloric acid in the alkaline aqueous layer, added 200ml of ...
example 3
[0030] Example 3: Synthesis of Valsartan
[0031] Add 200ml of water, 25g of sodium carbonate, 350ml of toluene and 28g of condensate hydrochloride into the reaction bottle, stir to dissolve, cool down to 10°C, add 18g of valeryl chloride dropwise, and keep it warm at 10°C for 2 hours. After the reaction is complete, let it stand The layers were separated, and the lower aqueous layer was separated. The organic layer was washed with sodium bicarbonate solution and dilute hydrochloric acid, dried over magnesium sulfate, and filtered to obtain a pentanoylation reaction solution. Add 11g of sodium azide and 23.5g of triethylamine hydrochloride to the valerylation reaction solution, reflux for 25 hours, cool down to 50°C, add 200ml of 8% sodium hydroxide solution, react at 20°C for 4 hours, and let stand Separate the layers, separate the organic layer, adjust the pH of the reaction solution to 1-2 with 6M dilute hydrochloric acid in the alkaline aqueous layer, add 200ml of isopropy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com