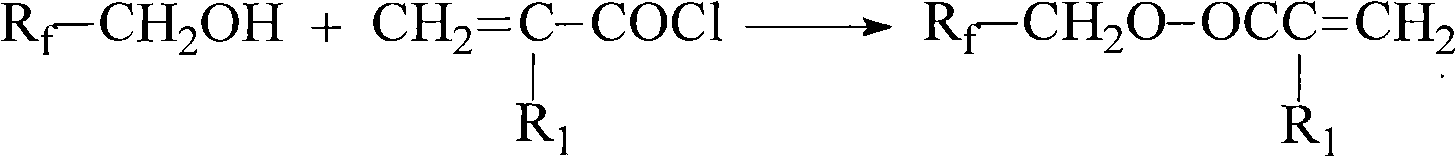Method for preparing fluorine-containing acrylate
A technology of acrylates and polyfluoroalcohols, which is applied in the preparation of carboxylic acid halides, organic chemistry, textiles and papermaking, etc. It can solve the problems of decreased waterproof and oil-proof performance of copolymers, increased surface energy, and decreased receding dynamic contact angles. , to achieve excellent water and oil repellency, non-bioaccumulation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
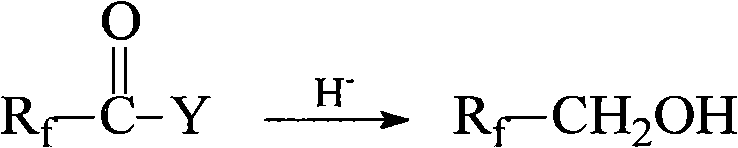
[0029] Preparation of 2,2,3,3,4,4,4-heptafluorobutyl acrylate
[0030] Slowly dissolve 7.57g (0.200mol) of sodium borohydride into 71.34g of anhydrous ether at 5-10°C, then slowly add 21.41g (0.100mol) of perfluorobutyric acid dropwise. The reaction was stirred at ~-5°C for 10 h. Then stir while slowly adding 10.0g of water to remove excess sodium borohydride (the amount of water is greater than the amount of reducing agent used, less than 1 / 5 of the total organic phase), this time the liquid into a colloidal substance, then dropwise added 20.0g 10% sodium hydroxide aqueous solution, the colloidal substance slowly forms a granular solid, and the whole process emits a large amount of heat. Keep the system temperature at 5-10 ° C, stir for 30 min and then filter. The filtrate is concentrated in a room temperature bath with a rotary evaporator , to get the crude product, then rectification to obtain 16.59g 2,2,3,3,4,4,4-heptafluoro-1-butanol CF 3 CF 2 CF 2 CH 2 OH, yield 82....
Embodiment 2
[0033] Preparation of 1H,1H-perfluorohexyl methacrylate
[0034] Slowly dissolve 5.68g (0.150mol) of lithium aluminum hydride into 106.72g of anhydrous tetrahydrofuran at 0-5°C, and slowly add 29.28g (0.088mol) of perfluorohexanoyl chloride dropwise. The reaction was stirred at ℃ for 8h. Then slowly add 10.0 g of water under stirring, then add 20.0 g of 10% sodium hydroxide aqueous solution, keep the system temperature at 5-10° C. and stir for 20 min, then filter, and the filtrate is concentrated in a room temperature bath with a rotary evaporator to obtain a crude product. Then rectification gives 22.93g 1H, 1H-perfluoro-1-hexanol CF 3 (CF 2 ) 4 CH 2 OH, yield 86.8%.
[0035] 21.14g (0.070mol) 1H, 1H-perfluoro-1-hexanol, 10.11g triethylamine, 49.95g ether and 0.011g phenothiazine were added in the flask with condenser. Cool and keep the temperature in the flask as At 0°C, 9.70 g (0.093 mol) of methacryloyl chloride was added dropwise. After the dropwise addition, stir ...
Embodiment 3
[0037] Preparation of 1H,1H-2-trifluoromethyl-3-oxaperfluorohexyl acrylate
[0038] Slowly dissolve 3.63g (0.096mol) of lithium aluminum hydride into 92.94g of dioxane at -5 to 0°C, and slowly add 19.58g (0.059mol) of 2-trifluoromethyl-3-oxa Fluorocaproyl fluoride, after the dropwise addition, stir and react at 0-5°C for 6h, then slowly add 10.0g of water under stirring, then add 20.0g of 10% sodium hydroxide aqueous solution, keep the system temperature at 5-10°C, and stir for 15min After filtration, the filtrate was concentrated with a rotary evaporator in a room temperature bath to obtain a crude product, which was then rectified to obtain 16.82 g of 1H, 1H-2-trifluoromethyl-3-oxaperfluoro-1-hexanol CF 3 CF 2 CF 2 OCF (CF 3 )CH 2 OH, yield 89.3%.
[0039] Dissolve 16.09g (0.050mol) of 1H, 1H-2-trifluoromethyl-3-oxaperfluoro-1-hexanol, 7.574g (0.062mol) of N,N-dichloromethane in 79.62g of dichloromethane Add methylaniline and 0.008g of phenothiazine into a flask with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com