Method for continuously synthesizing ethyl 4-chloroacetoacetates
A technology of chloroacetoacetic acid and esters, which is applied in the preparation of acyl halides, carboxylic acid halides, organic chemistry, etc., can solve the problems of small effective cooling area, difficult subsequent separation, low utilization rate of equipment, etc., and achieve a solution to the reaction temperature Low temperature, controlled reaction temperature, and easy rectification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
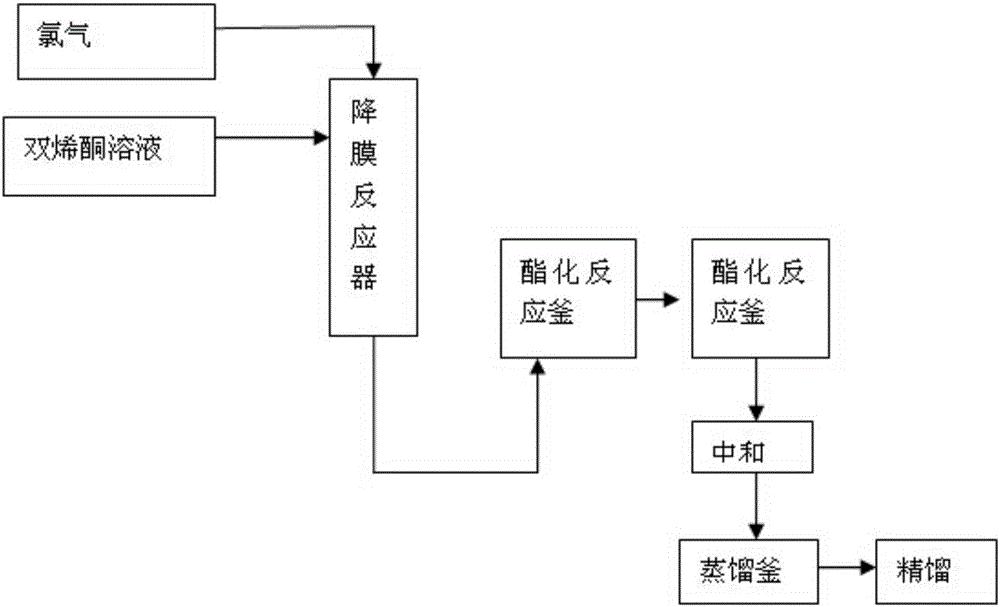
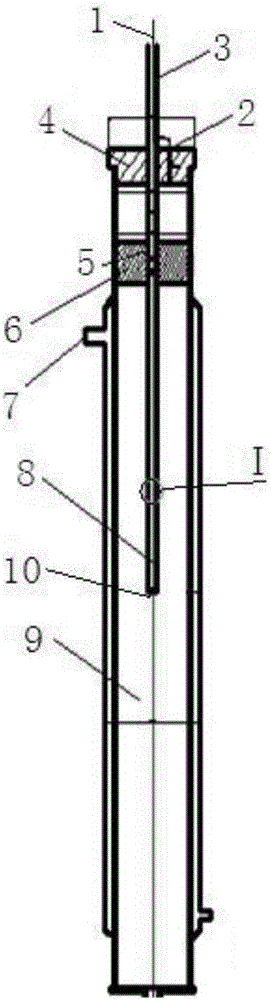
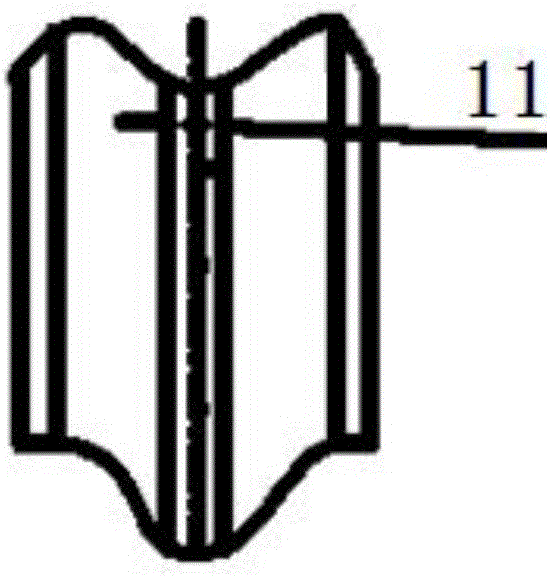
[0029] Diketene is mixed with dichloromethane to prepare a solution containing about 7.68-7.70% of diketene, cooled to -5~0℃, and the configured diketene solution enters through a Teflon distributor (30mm above the distributor) -In the falling film reactor cooled by the refrigerant at 5~0℃, the flow rate is 50ml / min, the chlorine gas enters the reactor through the gas distribution pipe (polytetrafluoroethylene, opening number 40, pore diameter 0.4mm), flow rate 75L / h; diene The molar ratio of ketone to chlorine is 1.00:0.97 (±0.02); the gas phase such as unreacted chlorine enters the tail gas absorber; after the reaction liquid flows out of the reactor, it enters the esterification reactor through a gas-liquid separator.
[0030] The chlorination reaction liquid enters the esterification reactor and is mixed with ethanol for esterification reaction. The temperature of the esterification reaction is controlled at 5~10℃; the amount of ethanol added is controlled at diketene: ethanol...
Embodiment 2
[0032] Diketene is mixed with dichloromethane to prepare a solution containing about 5% of diketene, cooled to -15~-10℃, and the configured diketene solution enters through the distributor (nickel, the upper liquid layer of the distributor 40mm) In the falling film reactor cooled by the refrigerant at -15~-10℃, the flow rate is 77ml / min, and the chlorine gas enters the reactor through the gas distribution pipe (nickel tube, opening 60, aperture 0.1mm), and the flow rate is 79L / h. The molar ratio of diketene to chlorine is 1.00:1.02. Unreacted chlorine and other gaseous phases enter the tail gas absorber; after the reaction liquid flows out of the reactor, it enters the esterification reactor through a gas-liquid separator.
[0033] The chlorination reaction liquid enters the esterification reactor and is mixed with methanol for esterification reaction. The esterification reaction temperature is controlled at -10~0℃; the amount of methanol added is controlled at diketene: methanol...
Embodiment 3
[0035] Diketene is mixed with chloroform to prepare a solution containing about 30% of diketene, cooled to 10~15℃, and the configured diketene solution enters through the distributor (Hastelloy, the upper liquid layer of the distributor 20mm) In a falling film reactor cooled by a refrigerant at 10 to 15°C, the flow rate is 12ml / min, and the chlorine gas enters the reactor through a gas-phase distribution pipe (Hastelloy, 20 openings, 1mm diameter), and a flow rate of 65L / h. The molar ratio of diketene to chlorine is 1.00:0.85. Unreacted chlorine and other gaseous phases enter the tail gas absorber; after the reaction liquid flows out of the reactor, it enters the esterification reactor through a gas-liquid separator.
[0036] The chlorination reaction liquid enters the esterification reactor and is mixed with isopropanol for esterification reaction. The temperature of the esterification reaction is controlled at 10-15°C; the amount of isopropanol added is controlled at diketene: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com