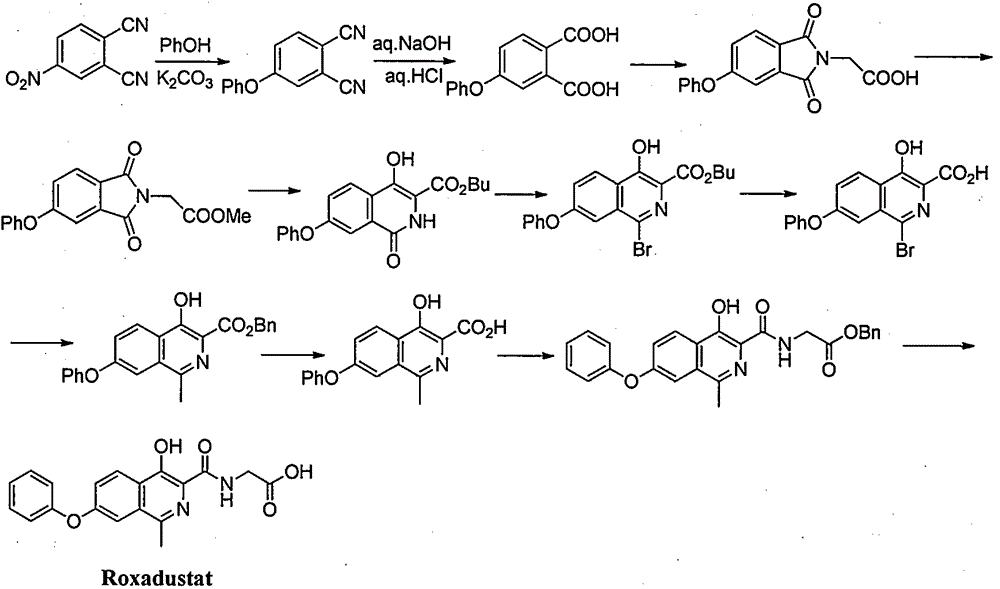
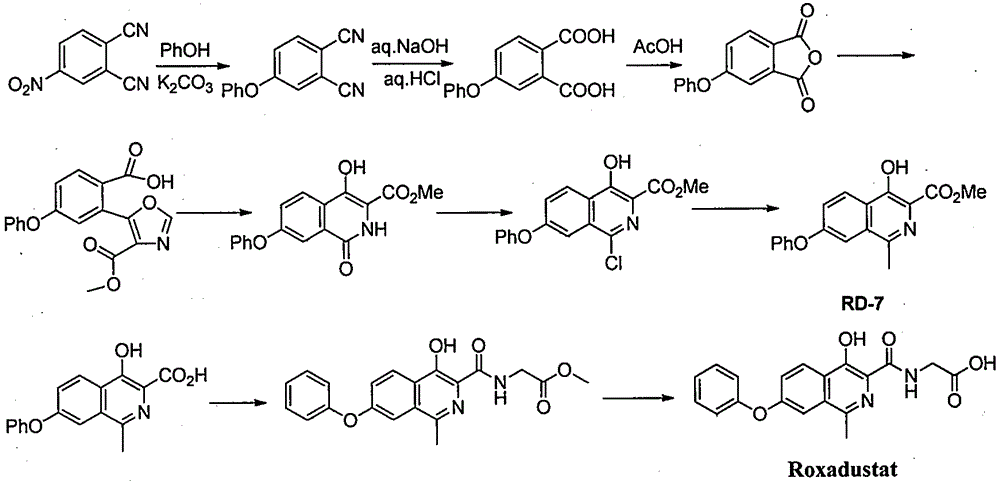
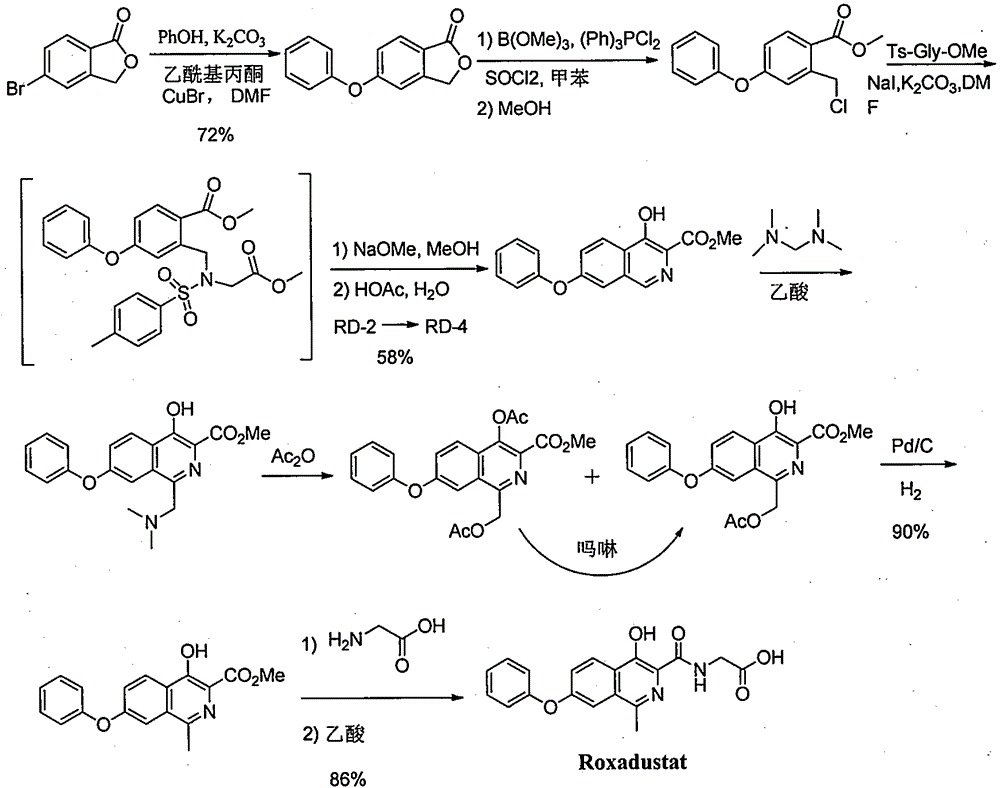
Preparation method for intermediate of Roxadustat
A technology for intermediates and compounds, which is applied in the preparation of acyl halides, carboxylic acid halides, sulfonamides, etc., can solve the problems of difficult quality purification of final product raw materials, cumbersome preparation process, and high preparation costs, and achieve operational Convenience, high reaction yield, easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of 3-methyl-5-bromoisobenzofuran-1(3H)-one (I)
[0050]
[0051] In a 3L reaction flask, 2-hydroxy-5-bromoacetophenone (215g, 1mol), ethyl carbazate (104g, 1mol) and absolute ethanol (1.5L) were sequentially added. Heated to reflux and stirred for 5h, cooled to room temperature, and solid precipitated out. After filtering, the filter cake was washed with absolute ethanol (0.3 L), and dried in vacuum (50° C.) for 5 h to obtain Intermediate 3 (247.1 g, yield 82.1%).
[0052] In a 3L reaction flask, Intermediate 3 (245g, 0.8mol) was dissolved in dichloromethane (2L), at room temperature, iodophenyldiethyl ester (386g, 1.2mol) was added in batches within 10min, after the addition was complete, stir at room temperature Reaction 4h. The reaction solution was extracted with water (1 L), and the organic layer was concentrated to dryness under reduced pressure to obtain the crude intermediate 5, which was directly put into the next step without purification.
...
Embodiment 2
[0055] Preparation of 3-methyl-5-phenoxyisobenzofuran-1(3H)-one (II)
[0056]
[0057] In a 2L reaction flask, add phenol (188g, 2mol), 3-methyl-5-bromoisobenzofuran-1(3H)-one (1, 227g, 1mol), cuprous bromide (21.7g, 0.5 mol), acetylacetone (10 g, 0.1 mol), potassium carbonate (276 g, 2 mol) and N,N-dimethylformamide (1 L). Heated to an internal temperature of 120°C for 10 h, cooled to room temperature, poured the reaction solution into 2N cold aqueous hydrochloric acid (1.5 L), and stirred for 30 min. After filtration, the filter cake was washed with water (0.5 L), and dried in vacuum (60° C.) for 5 h to obtain the compound of formula II (197 g, yield 82%). MS m / z 241[M+H] + .
Embodiment 3
[0059] Preparation of 3-methyl-5-phenoxyisobenzofuran-1(3H)-one (II)
[0060] In a 2L reaction flask, add phenol (188g, 2mol), 3-methyl-5-bromoisobenzofuran-1(3H)-one (1, 227g, 1mol), cuprous chloride (49.5g, 0.5 mol), acetylacetone (10 g, 0.1 mol), cesium carbonate (652 g, 2 mol) and N-methylpyrrolidone (1 L). Heated to an internal temperature of 120°C for 10 h, cooled to room temperature, poured the reaction solution into 2N cold aqueous hydrochloric acid (1.5 L), and stirred for 30 min. After filtering, the filter cake was washed with water (0.5 L), and dried in vacuum (60° C.) for 5 h to obtain the compound of formula II (194 g, yield 80.8%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com