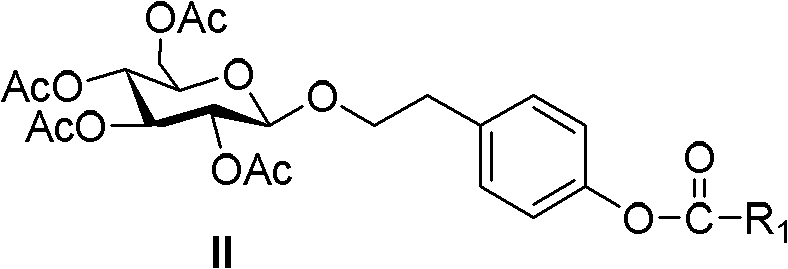
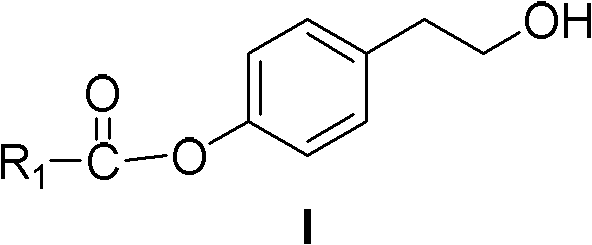
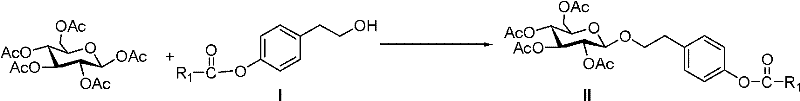Salidroside chemical synthesis method for industrialization
A salidroside and compound technology, applied in chemical instruments and methods, organic chemistry, bulk chemical production, etc., can solve the problems that product separation must rely on column chromatography, process industrialization amplification, and complex reaction products, etc., to achieve good results Effects of crystallinity, reduction of contamination, and simplification of purification operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of 2-(4-benzoyloxyphenyl) ethanol
[0044] Dissolve tyrosol (1kg, 7.24mol) in 10L ethyl acetate, add 1.2L (1.2eq) triethylamine, and stir in an ice bath; dissolve 1L (1.2eq) of benzoyl chloride in 2L ethyl acetate, add dropwise into the above solution. After dropping, react at room temperature for 1 h. The reaction solution was filtered, washed three times with a saturated aqueous solution of sodium carbonate and three times with a saturated aqueous solution of sodium chloride, dried over anhydrous sodium sulfate, and concentrated. Petroleum ether: chloroform=5:1 recrystallized, filtered and dried. 1.23 kg of white scaly solid was obtained, with a yield of 70.1%. The melting point is 66.2-67.9°C.
[0045] 1 H NMR (400MHz, CDCl 3 )δ8.20(d, J=7.7Hz, 2H), 7.63(t, J=7.4Hz, 1H), 7.51(t, J=7.7Hz, 2H), 7.28(d, J=8.4Hz, 2H) , 7.16(d, J=8.4Hz, 2H), 3.86(t, J=6.5Hz, 2H), 2.88(t, J=6.6Hz, 2H), 1.65(br, 1H). 13 C NMR (100MHz, CDCl 3 )δ165.46, ...
Embodiment 2
[0046] Embodiment 2: the preparation of 2-(4-benzoyloxyphenyl) ethanol
[0047]Tyrosol (1kg, 7.24mol) was dissolved in 10L ethyl acetate, 1.2L (1.2eq) triethylamine was added, stirred under ice bath; benzoyl chloride 1L (1.2eq) was dissolved in 2L ethyl acetate, drop Add to the above solution. After dropping, react at room temperature for 1 h. The reaction solution was filtered, washed three times with a saturated aqueous solution of sodium carbonate and three times with a saturated aqueous solution of sodium chloride, dried over anhydrous sodium sulfate, and concentrated. Recrystallized from isopropyl ether, filtered and dried. 1.3 kg of white scaly solid was obtained, with a yield of 74.1%. The melting point is 66.3-67.9°C.
Embodiment 3
[0048] Embodiment 3: the preparation of 2-(4-benzoyloxyphenyl) ethanol
[0049] Dissolve tyrosol (1kg, 7.24mol) in 10L ethyl acetate, add 1.2L (1.2eq) triethylamine, stir under ice bath; dissolve 1.04L (1.2eq) of benzoyl bromide in 2L ethyl acetate , added dropwise to the above solution. After dropping, react at room temperature for 1 h. The reaction solution was filtered, washed three times with a saturated aqueous solution of sodium carbonate and three times with a saturated aqueous solution of sodium chloride, dried over anhydrous sodium sulfate, and concentrated. Recrystallized from isopropyl ether, filtered and dried. 1.25 kg of white scaly solid was obtained, with a yield of 71.3%. The melting point is 66.1-67.9°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com