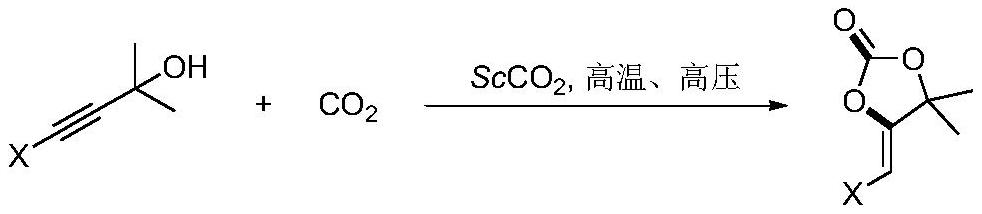
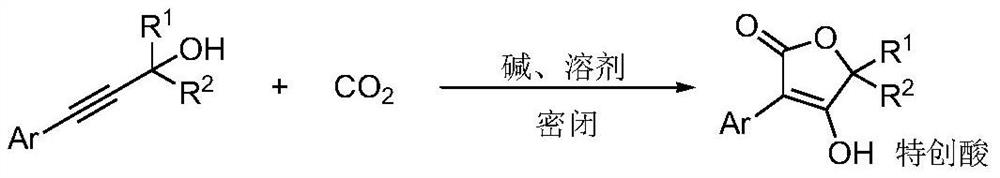
Method for preparing tetronic acid by cyclization of propargyl alcohol and carbon dioxide
A technology of carbon dioxide and propargyl alcohol, applied in the field of chemistry, can solve the problems of harsh reaction conditions, improved reaction selectivity, difficult preservation and the like, and achieves the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
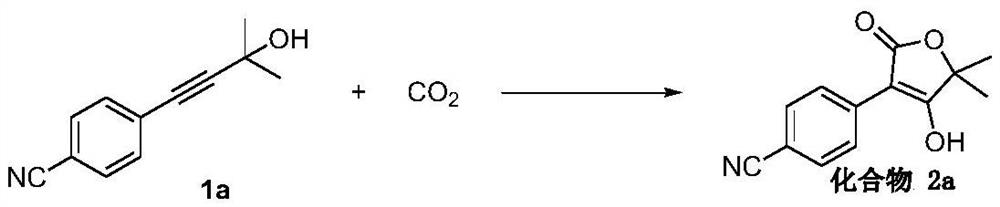
[0038] Example 1 Synthesis of 5,5-dimethyl-4-hydroxyl-3-(4-cyanophenyl)-2(5H)-furanone (compound 2a)
[0039]
[0040] Accurately measure 4-(4-cyano-phenyl)-2-methyl-3-butyn-2-ol 1a (74.1 mg, 0.4 mmol) into the sealed tube, and weigh K under nitrogen 2 CO 3 (442.3 mg, 3.2 mmol), 2 mL of acetonitrile was added, and carbon dioxide was introduced. Under normal pressure, react at 25°C for 8 hours, cool to room temperature, quench with 2M hydrochloric acid solution, extract with dichloromethane, concentrate the organic phase, and perform column chromatography to obtain 84.4 mg (0.368 mmol) of a white solid product with a yield of 92%. .
[0041] 1 H NMR (400MHz, DMSO-d 6 ) δ8.39~8.33 (m, 2H), 7.68~7.56 (m, 2H), 1.35 (s, 6H).
Embodiment 2
[0042] Example 2 Synthesis of 5-methyl-5-ethyl-4-hydroxyl-3-(4-cyanophenyl)-2(5H)-furanone (compound 2b)
[0043]
[0044] Accurately measure 4-(4-cyano-phenyl)-3-methyl-4-pentyn-3-ol 1b (79.7mg, 0.4mmol) into the sealed tube, weigh DBU (487.2mg, 3.2mmol), add 3mL DMF (N,N-dimethylformamide), and pass through carbon dioxide. Under normal pressure, react at 25°C for 8 hours, cool to room temperature, quench with 2M hydrochloric acid solution, extract with dichloromethane, concentrate the organic phase, and perform column chromatography to obtain 82.7mg (0.340mmol) of a white solid product with a yield of 85% .
[0045] 1 H NMR (400MHz, DMSO-d 6 )δ8.47(d, J=8.4Hz, 2H), 7.49~7.47(m, 2H), 1.56(ddq, J=28.4, 14.3, 7.2Hz, 2H), 1.19(s, 3H), 0.71(t , J=7.4Hz, 3H).
Embodiment 3
[0046] Example 3 Synthesis of 5,5-diethyl-4-hydroxyl-3-(4-cyanophenyl)-2(5H)-furanone (compound 2c)
[0047]
[0048] Accurately measure 4-(4-cyano-phenyl)-3-methyl-4-pentyn-3-ol 1c (85.3 mg, 0.4 mmol) into the sealed tube, weigh CsF (486.1 mg, 3.2 mmol), 5 mL of DMI (1,3-dimethylimidazolidinone) was added, and carbon dioxide was introduced. Under normal pressure, react at 25°C for 8 hours, cool to room temperature, quench with 2M hydrochloric acid solution, extract with dichloromethane, concentrate the organic phase, and perform column chromatography to obtain 77.2 mg (0.300 mmol) of a white solid product with a yield of 75%. .
[0049] 1 H NMR (400MHz, DMSO-d6) δ8.18 (d, J = 8.2Hz, 2H), 7.79 (d, J = 8.2Hz, 2H), 1.86 (dq, J = 14.9, 7.2Hz, 4H), 0.73 (t, J=7.3Hz, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com