Methods and compositions for cell-proliferation-related disorders
a cell proliferation and composition technology, applied in the direction of drug compositions, diagnostic recording/measuring, instruments, etc., can solve problems such as imbalance, achieve optimal matching of subjects, reduce neoactivity, and significantly increase neoactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
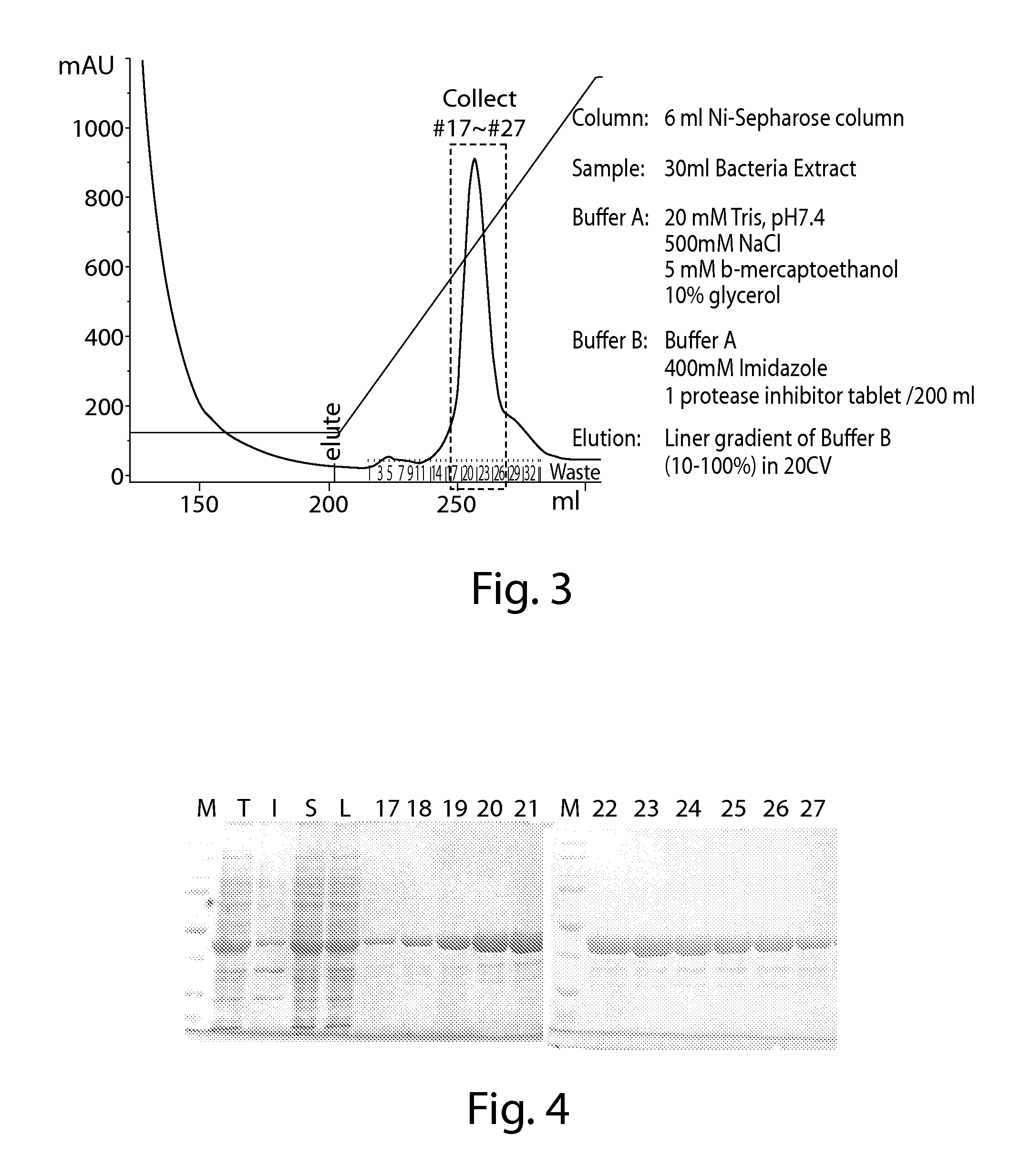
IDH1 Cloning, Mutagenesis, Expression and Purification
[0637]1. Wild Type IDH1 was Cloned into pET41a, Creating His8 Tag at C-Terminus.
[0638]The IDH1 gene coding region (cDNA) was purchased from Invitrogen in pENTR221 vector (www.invitrogen.com, Cat#B-068487_Ultimate_ORF). Oligo nucleotides were designed to PCR out the coding region of IDH1 with NdeI at the 5′ end and XhoI at the 3′. (IDH1-f: TAATCATATGTCCAAAAAAATCAGT (SEQ ID NO:1), IDH1-r: TAATCTCGAGTGAAAGTTTGGCCTGAGCTAGTT (SEQ ID NO:2)). The PCR product is cloned into the NdeI / XhoI cleaved pET41a vector. NdeI / XhoI cleavage of the vector pET41a releases the GST portion of the plasmid, and creating a C-terminal His8 tag (SEQ ID NO:3) without the N-terminal GST fusion. The original stop codon of IDH1 is change to serine, so the junction sequence in final IDH1 protein is: Ser-Leu-Glu-His-His-His-His-His-His-His-His-Stop (SEQ ID NO:4).
[0639]The C-terminal His tag strategy instead of N-terminal His tag strategy was chosen, because C-term...
example 2
Enzymology Analysis of IDH1 Wild Type and Mutants
1. Analysis of IDH1 Wild-Type and Mutants R132H and R132S in the Oxidative Decarboxylation of Isocitrate to α-Ketoglutarate (α-KG).
A. Methods
[0652]To determine the catalytic efficiency of enzymes in the oxidative decarboxylation of isocitrate to α-Ketoglutarate (α-KG) direction, reactions were performed to determine Vmax and Km for isocitrate. In these reactions, the substrate was varied while the cofactor was held constant at 500 uM. All reactions were performed in 150 mM NaCl, 20 mM Tris-Cl, pH 7.5, 10% glycerol, and 0.03% (w / v) BSA). Reaction progress was followed by spectroscopy at 340 nM monitoring the change in oxidation state of the cofactor. Sufficient enzyme was added to give a linear change in absorbance for 10 minutes.
B. ICDH1 R132H and ICDH1 R132S are impaired for conversion of isocitrate to α-KG.
[0653]Michaelis-Menten plots for the relationship of isocitrate concentration to reaction velocity are presented in FIGS. 12A-12...
example 3
Metabolomics Analysis of IDH1 Wild Type and Mutants
[0700]Metabolomics research can provide mechanistic basis for why R132 mutations confer survival advantage for GBM patients carrying such mutations.
1. Metabolomics of GBM Tumor Cell Lines: Wild Type Vs R132 Mutants
[0701]Cell lines with R132 mutations can be identified and profiled. Experiments can be performed in proximal metabolite pool with a broad scope of metabolites.
2. Oxalomalate Treatment of GBM Cell Lines
[0702]Oxalomalate is a competitive inhibitor of IDH1. Change of NADPH (metabolomics) when IDH1 is inhibited by a small molecule can be examined.
3. Metabolomics of Primary GBM Tumors: Wild Type Vs R132 Mutations
[0703]Primary tumors with R132 mutations can be identified. Experiments can be performed in proximal metabolite pool with a broad scope of metabolites.
4. Detection of 2-Hydroxyglutarate in Cells that Overexpress IDH1 132 Mutants
[0704]Overexpression of an IDH1 132 mutant in cells may cause an elevated level of 2-hydroxy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| imaging | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com