Water soluble nanoparticles of hydrophilic and hydrophobic active materials and an apparatus and method for their production
a technology of hydrophilic and hydrophobic active materials and water soluble nanoparticles, which is applied in the field of nanoparticles, can solve the problems of hindering the development of therapeutic agents, affecting the solubility of water, and each of the existing companies focusing on these large and small molecules has its own restriction and limitations, so as to achieve high purity, simplify the process, and ensure the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Procedure for the Production of the Inclusion Complex
[0103]A. Preparation of Polymer Solution.
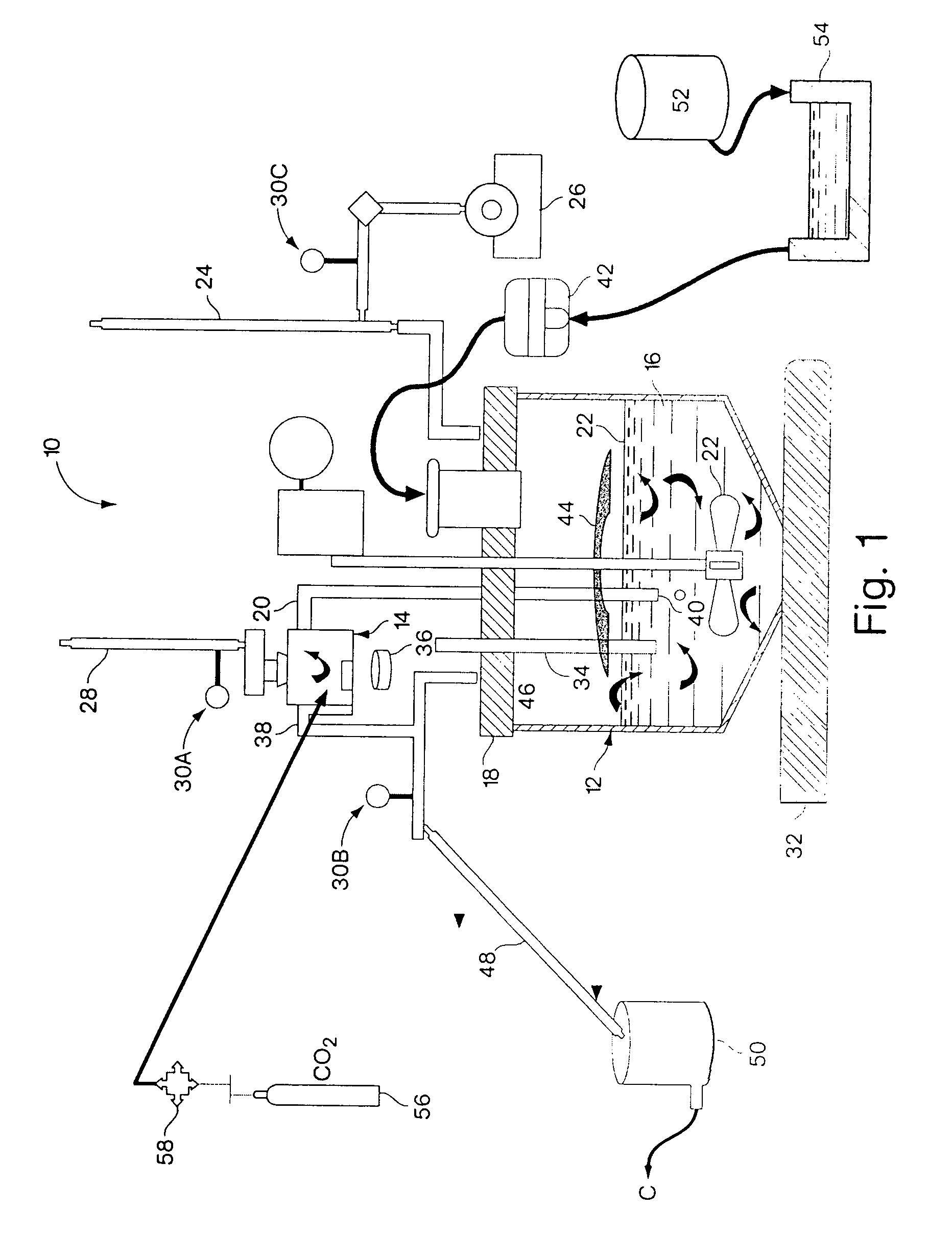
[0104]500 ml of distilled water are transferred from distilled water vessel 52 to polymer vessel 54. To polymer vessel 54 is added an estimated quantity of polymer, which was chosen for creation of Inclusion Complex with lipophilic compound. At temp. 20–25° C. the contents of polymer vessel 54 is mixed at velocity of 30–60 min−1 (rotation / min). up to complete dissolution of the polymer and creation of transparent or opalescent solution.
[0105]B. Loading Compounds in the Reactor
[0106]The polymer solution, prepared in polymer vessel 54 is transferred into first vessel 12 by pump 42. In the same vessel the carrier solvent is loaded. Lipophilic compound is placed into second vessel 14.
[0107]C. Starting the Reactor
[0108]The nano-disperser 22 is activated at velocity 500–800 min−1. The cooling water is entered into first and second condensers 24 and 28. The heater (thermostat) 32 is a...
example 2
Modification of Polysaccharide
[0116]Distilled water with polysaccharide in varying amount was put into the vessel. After that a citric acid was added until the designated pH 2 at mixing was attained. X1 signifies the amount of polysaccaride in water, X2 signifies the pH value of water solution of polysaccharide
[0117]The obtained suspension is heated for approximately 10–20 minutes with continuous mixing at room temperature up till to 70–95 degrees ° C. up till a homogeneous opaque mass is obtained. The obtained mass is put in an autoclave on time X3 and exposed in an autoclave at temperatures 160–180° C. Under these conditions the network structures of a polysaccharide partially or completely are transformed to linear weakly branched macromolecules and which dissolve in water. Upon termination of the autoclave time the cooling below 100° C. is effected and a solution of polymer is obtained. A solution of polyethylene glycol—400 (PEG-400) in an amount X4 (% in relation to polysacchar...
example 3
Creation of Solu Nano-particles Wrapped in Modified Polysaccharide (Parts by Weight)
[0119]In distilled water a polysaccharide is dissolved, initially heated at 160–180 degrees C. up to molecular masses (5–10)×10(4) and is modified by the polyethylene glycol PEG-400. Conditions of modification: ratio “polysaccharide—polyethylene glycol PEG-400” ratio from 2:1 up to 4:1, acidic environment with pH 2–5 created by a citric acid, temperature 160–180° C., time of modification 60–180 min. Solution of a modified polysaccharide is put in a reactionary vessel, heated up to 60° C. mixing by a homogenizer at speed 10,000 and up rev / min.
[0120]Simultaneously a solution of macrolide in an organic solvent is prepared. Allowing the solution of a polysaccharide to reach given temperature 60° C., then it start to add a solution of macrolide with speed about 1 ml / sec. Speed of a homogenizer is increased to 10 thousand rev / min and up. The macrolide interacts with the polymer creating nanoparticles, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| sizes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com