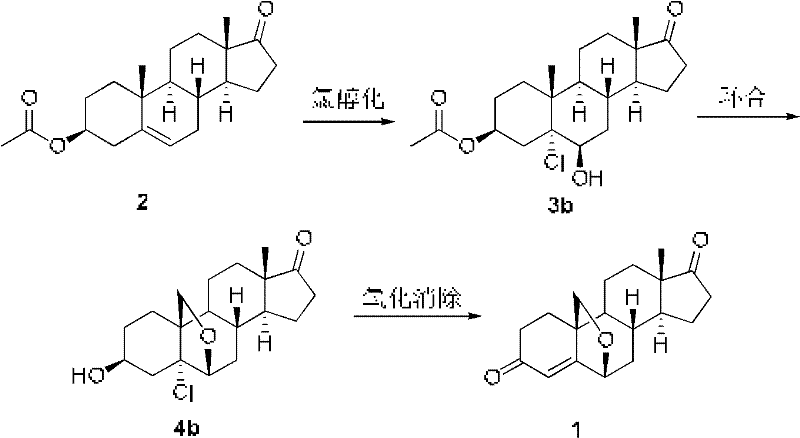
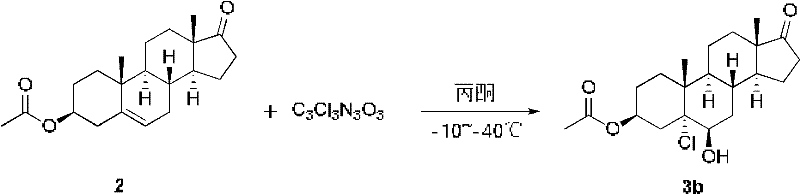Method for preparing compound 6beta, 19beta-epoxy-4-androstene-3, 17-diketone
A compound and androstene technology, which is applied in the field of preparation of compound 6β,19β-epoxy-4-androstene-3,17-dione, can solve the problems of high cost and large usage amount, and achieve cost saving , the effect of improving the reaction speed, improving product quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of the compound shown in formula 3a
[0031]
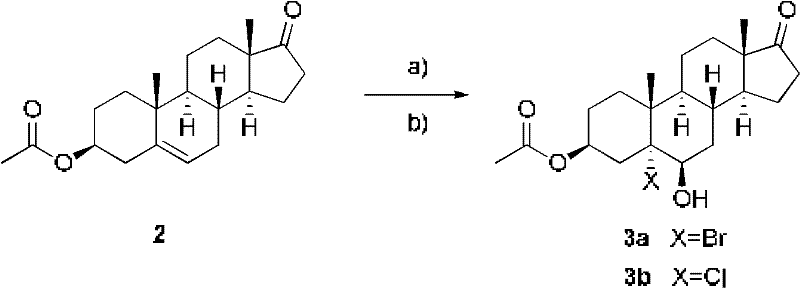
[0032] Add 16.5g compound 3β-acetoxy-androst-5-en-17-one (formula 2) (purchased from Zhejiang Shenzhou Pharmaceutical Co., Ltd.) into the reaction kettle, stir with 125mL dioxane and 25mL water Dissolve and cool down to 0°C. Slowly add 2.5mL of 1N perchloric acid and control the temperature at 0°C. Add 7.59g of N-bromoacetamide (NBA) in batches, react at 0°C, and follow the complete reaction by TLC. Pour into ice water, extract with chloroform, wash twice with 10% sodium sulfite 100mL, wash with water, concentrate under reduced pressure, centrifuge, add 500mL ethyl acetate to the wet product, reflux with 0.8g activated carbon, filter, concentrate under reduced pressure, centrifuge, and dry About 12.7 g of bromoalcoholate 3a was obtained, and the yield was about 60%.
[0033] mp: 174-176°C; EI MS (70eV, m / z): 428 (M + , 10%); 1 H NMR (300MHz, CDCl 3)δ5.44-5.48(m, 1H, 3-H), 4.23-4.33(m, ...
Embodiment 2
[0034] Embodiment 2: the preparation of the compound shown in formula 3b
[0035]
[0036] Add 30 g of compound 3β-acetoxy-androst-5-en-17-one (Formula 2) into the reaction kettle, stir and heat the solution with 600 mL of acetone, and cool down to -30°C. Slowly add 50% acetic acid (82.5 mL) and control the temperature at -30°C. Calcium hypochlorite (41 g in total) was added in batches, and after the addition was completed and the insulation reaction was completed, the reaction was followed by TLC. About 150 mL of 10% sodium sulfite was added dropwise to stop the reaction. Concentrate the acetone under reduced pressure, add 900mL ice water, stir for half an hour, centrifuge, rinse with water until neutral, and shake dry. Add 900 mL of ethyl acetate to the wet product, reflux 1.5 g of activated carbon, filter, concentrate under reduced pressure, centrifuge, and dry to obtain about 24 g of chloroalcohol compound 3b, with a yield of about 70%.
[0037] mp: 224-226°C; EI MS ...
Embodiment 3
[0038] Embodiment 3: the preparation of the compound shown in formula 4a
[0039]
[0040] Add 20 g of bromoalcoholate 3a, 400 mL of cyclohexane, 12 g of anhydrous sodium carbonate, 5 g of iodine, 11 g of N-chlorosuccinimide (NCS), and 1.6 g of benzoyl peroxide. Illumination, heating and reflux. TLC followed the reaction. After the reaction was complete, the temperature was lowered, and 100 mL of 5% sodium thiosulfate was added to wash, washed with water, and concentrated. Subsequently, 100 mL of methanol and 15 mL of aqueous solution of 4 g of potassium carbonate were added, and the temperature was raised to reflux until the reaction was complete. Concentrate under reduced pressure, add water and concentrate until thick, cool down, stir, centrifuge, and dry to obtain 14.3 g of cyclized product, with a yield of about 80%.
[0041] mp: 170-172°C; EI MS (70eV, m / z): 383 (M + , 50%); 1 H NMR (300MHz, CDCl 3 )δ4.01(d, 1H, 6-H), 3.98(m, 1H, 3-H), 3.88(d, 1H, 19-H a ), 3.86...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com