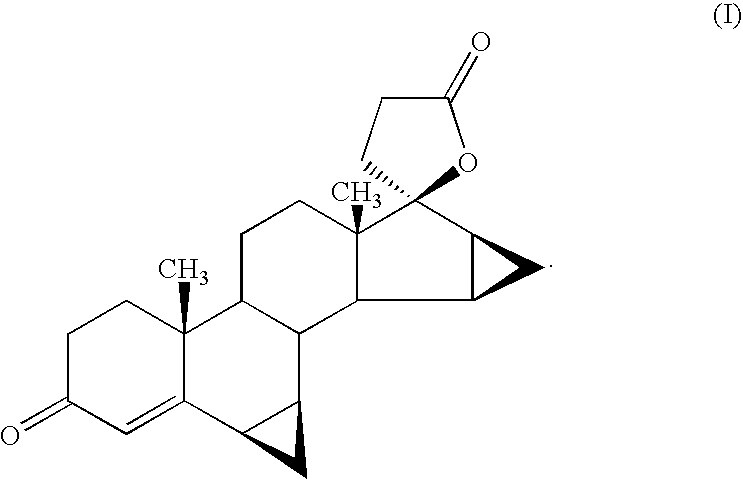
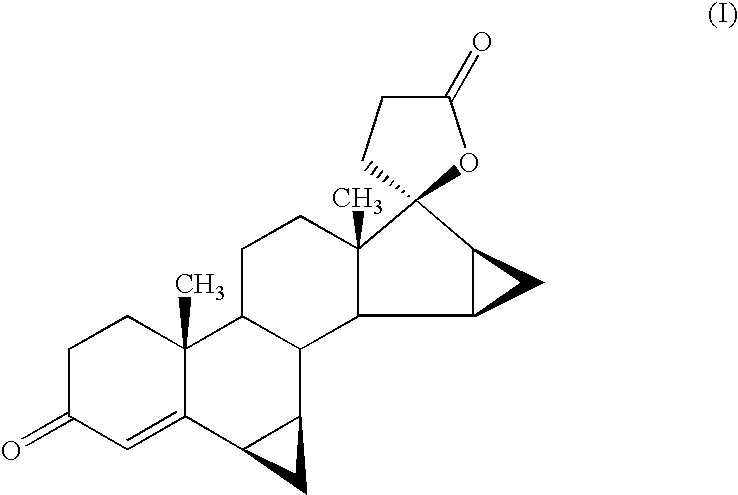
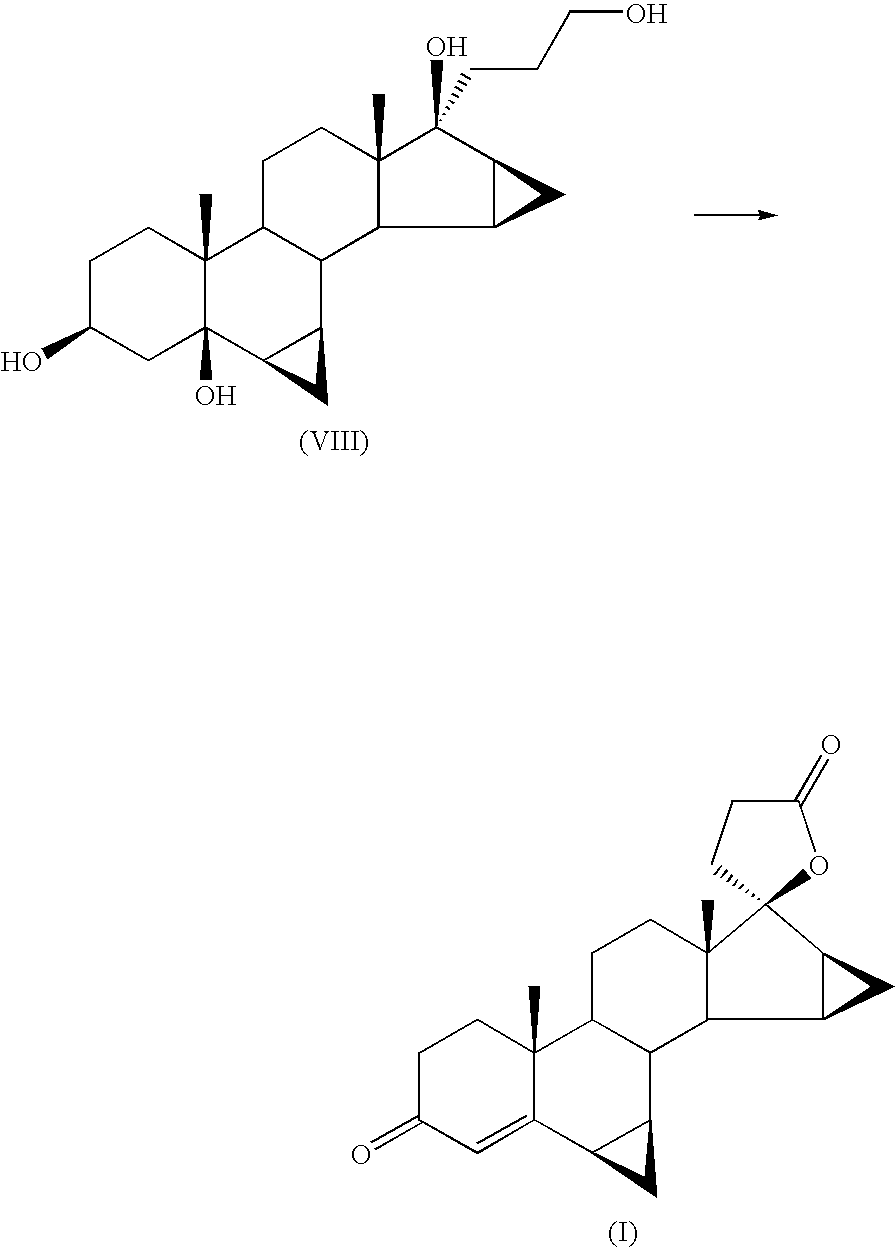
Process For The Preparation Of Drospirenone
a technology of drospirenone and process, which is applied in the field of industrial scale preparation of drospirenone, can solve the problems of large quantity and practically unusability of highly toxic solvents, and achieve the effect of high final purity and high degree of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 7α-bromo-5,6β-epoxy-15β,16β-methylene-3β-pivaloyloxy-5β-androstan-17-one
Step a)
[0036]67.5 g of 5,6β-epoxy-7β-hydroxy-15β,16β-methylene-3β-pivaloyloxy-5β-androstan-17-one are dissolved in 205 ml of pyridine in a 2 litre flask, under nitrogen.
[0037]17.5 ml of mesyl chloride are added from a dropping funnel, maintaining a temperature of 20 / 25° C.
[0038]The mixture is stirred for 1 hour at 20° C. to obtain a thick orange suspension.
[0039]The progress of the reaction is checked by TLC. Once the reaction is completed, 83.2 g of lithium bromide dissolved in 54 ml of water are added and the temperature is brought to 70 / 75° C. After 3 hours another 8 g of lithium bromide dissolved in water and 50 ml of pyridine are added.
[0040]At the end of the reaction (checked by TLC) the temperature is brought to 60° C. and 700 ml of water are added; it is left to cool to 15 / 20° C., maintaining under stirring for 1 hour at this temperature.
[0041]The solid is filtered off and washed with 500 ...
example 2
Preparation of 5-hydroxy-15β,16β-methylene-3β-pivaloyloxy-5β-androst-6-en-17-one
Step b)
[0046]27 g of powdered zinc suspended in 91 ml of THF (tetrahydrofuran) are fed into a 1 litre flask, under nitrogen.
[0047]A solution of 67.5 g of 7α-bromo-5,6β-epoxy-15β,16β-methylene-3β-pivaloyloxy-5β-androstan-17-one, prepared as described in Example 1, in 277 ml of THF is then added; 19.9 ml of glacial acetic acid are slowly added dropwise, maintaining the temperature below 60° C. during the addition. The reaction mixture is maintained under stirring for 3 hours at 59 / 60° C.
[0048]At the end of the reaction (checked by TLC) and after cooling to 50° C., the zinc is filtered off over dicalite and the filter washed with 200 ml of THF.
[0049]The filtered solution is brought to pH 9 with 60 ml of triethylamine.
[0050]The solution is concentrated under reduced pressure at 50° C. to obtain about 180 g of a semi-solid product which is dissolved in 500 ml of a 5% acetic acid-water solution (pH=4 with a pr...
example 3
Preparation of 3β,5-dihydroxy-15β-16β-methylene-5β-androst-6-en-17-one
Step c)
[0056]43 g of 5-hydroxy-15β,16β-methylene-3β-pivaloyloxy-5β-androst-6-en-17-one prepared as described above in Example 2, 430 ml of THF, 215 ml of methanol and 12.9 g of potassium hydroxide are fed into a 2 litre flask, under nitrogen at 20° C. The suspension is stirred at 20° C. for 3 hours.
[0057]At the end of the reaction (checked by TLC), the reaction mixture is poured into 2 litres of water, brought to pH 7 with 20% sulphuric acid (about 25 ml) then the suspension is stirred for 1 hour at 0 / 5° C. The solid is filtered off, washed with water and dried for 12 hours under reduced pressure at 50° C. to obtain 30.6 g of the title compound.
[0058]The analytical data obtained for a sample purified by chromatography correspond to those given in EP 0 075 189.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com